February Research and News 2023
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney February Research and News.
February Research and News
Inflammation mediates the association between high dietary acid load and the progression of CKD
In a study published ahead of print in the Journal of Renal Nutrition, investigators looked at the link between dietary acid load, serum albumin, and the progression of CKD in 188 post-menopausal women over a period of 36 months.
Dietary acid load was estimated from potential renal acid load calculated using urinary measures of chloride, phosphate, sodium, potassium, calcium, and magnesium (UPRAL). Serum albumin levels were used as a marker of inflammation.
A 25 mEq/day increase in UPRAL was inversely associated with serum albumin and a 17% risk of decline in kidney function.
Mediation analysis indicated that the drop in serum albumin was responsible for 34% of the association between dietary acid load and the decline in kidney function.
The study suggests that inflammatory response might be a potential pathway explaining the effects of systemic acid load on the progression of kidney disease.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
A classic conventional research study found that magnesium supplementation did not slow vascular calcification in CKD
In this study that was published ahead of print in the journal of the American Society of Nephrology, investigators isolated the benefit of magnesium supplementation on vascular calcifications in CKD.
They enrolled 148 patients with an eGFR between 15 mL/min and 45 mL/min and randomly assigned them to receive oral magnesium hydroxide 15 mmol twice daily or a matching placebo for 12 months.
Then they looked at the coronary artery calcification (CAC) score after 12 months and adjusted for baseline CAC score, age, and diabetes mellitus.
Median eGFR was 25 mL/min at baseline, and median baseline CAC scores were 413 and 274 in the magnesium and placebo groups, respectively.
Despite plasma magnesium increasing significantly during the trial in the magnesium group, the baseline-adjusted CAC scores did not differ significantly between the two groups after 12 months.
The problem with this study is the following:
1. The baseline CAC was significantly higher in the magnesium group than in the placebo group. It is possible that that group had a higher risk and inflammation than the placebo group.
2. Magnesium doesn't work alone. The study should also assess other variables such as vitamin D, K, Calcium, and phosphorus, among others.
3. The increased deaths in the magnesium group could be just due to the higher baseline CAC.
4. Twelve months are not enough to study a process that takes years.
Can you think of other issues with this study? Email us at info@inkidney.com
Gender-affirming hormone therapy may affect measures of kidney function
This systematic review and meta-analysis of 26 studies published in CJASN indicated that gender-affirming hormone therapy might increase blood levels of creatinine (indicating potential kidney dysfunction or simply a change in lean muscle mass) in transgender men.
At 12 months after initiating gender-affirming hormone therapy, blood levels of creatinine increased by 0.15 mg/dL in transgender men and decreased by -0.05 mg/dL in transgender women.
No study reported the impact of gender-affirming hormone therapy on other markers or measures of kidney function.
An accompanying editorial notes that studies are needed to investigate the mechanism through which gender-affirming hormone therapy is associated with changes in creatinine and whether it independently affects kidney function.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
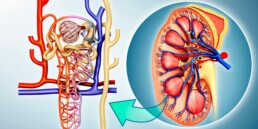
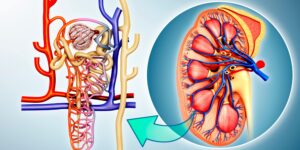
Mercury is toxic to the kidneys
Mercury is a toxic heavy metal that is widely distributed in the environment, and chronic exposure to it can cause serious health problems. One of the organs that are most susceptible to mercury toxicity is the kidneys. In this blog, we will discuss how chronic exposure to mercury is toxic to the kidneys.
Mercury is toxic to the kidneys
By Majd Isreb, MD, FACP, FASN, IFMCP
Sources of Mercury Exposure
Mercury can be found in various forms in the environment, including elemental (metallic) mercury, inorganic mercury, and organic mercury.
- Elemental mercury is commonly used in dental fillings, thermometers, and certain electrical equipment, and it can vaporize and be inhaled into the lungs.
- Inorganic mercury is used in the production of batteries, fungicides, and certain chemicals, and it can be absorbed through the skin and ingested through contaminated food and water.
- Organic mercury, also known as methylmercury, is the most toxic form of mercury and can accumulate in fish and other seafood, which are common food sources for many people.
The effects of mercury on human health depend on the following factors:
- Chemical form
- Dose
- Age or the developmental stage of the person exposed
- Duration of exposure
- Route of exposure route, including inhalation, ingestion, and dermal contact.
The body’s handling of mercury
According to the CDC, most participants tested in the NHANES 2003-2004 cohort had measurable mercury in their blood and urine. These levels increased with age.
Inhalation of elemental mercury leads to rapid absorption and distribution in all major organs. However, inorganic mercury compounds accumulate mainly in the kidneys after ingestion. The proximal tubules are the major site of accumulation of these compounds in the kidneys.
Methylmercury is a stable compound in the human body that is slowly transformed into other forms of mercury. It can cross the placenta and the blood-brain barrier and has a long half-life.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Mercury is toxic to the kidneys
The kidneys are particularly susceptible to mercury toxicity because they are responsible for filtering waste products from the blood and excreting them in the urine. Chronic exposure to mercury can cause damage to the kidneys, leading to various kidney-related health problems. Some of the effects of mercury toxicity on the kidneys include:
- Tubular injury: Mercury can cause tubular necrosis, which is the death of the tubular cells in the kidneys. This can result in a reduction in the ability of the kidneys to filter waste products from the blood, leading to the buildup of toxins in the body.
- Interference with kidney enzymes: Mercury can interfere with the normal functioning of kidney antioxidant enzymes, which can increase exposure to oxidative stress and the risk of kidney disease.
- Glomerular injury (glomerulonephritis): Mercury can cause injury to the glomerular cells in the kidneys. Several studies have shown that mercury can lead to minimal change disease and membranous nephropathy, two types of glomerulonephritis.
The role of genetics
The role of genetics in mercury toxicity and its effects on the kidneys is not fully understood. However, some studies suggest that genetics may play a role in determining an individual's susceptibility to mercury toxicity.
For example, genetic variations in certain genes involved in the transport and excretion of mercury from the body have been shown to affect the ability of an individual to eliminate mercury from their body, increasing their risk of toxicity.
Additionally, some genetic variations have been associated with increased susceptibility to nephrotoxicity and kidney damage caused by mercury exposure. For example, genetic variations in genes involved in oxidative stress and inflammation have been linked to an increased risk of kidney damage caused by mercury exposure.
It is important to note that while genetics may play a role in determining an individual's susceptibility to mercury toxicity and its effects on the kidneys, it is not the only factor. Other factors, such as the amount and duration of mercury exposure, lifestyle factors, and environmental factors, can also impact an individual's risk of toxicity.
The role of nutrition
Nutrition plays a crucial role in mitigating the effects of mercury toxicity on the kidneys. Adequate intake of certain nutrients, such as antioxidants and essential minerals, can help to reduce oxidative stress and inflammation in the kidneys, which can be caused by mercury exposure.
In addition, a healthy diet that is rich in antioxidants, vitamins, and minerals can help to support the overall health of the kidneys, which can be damaged by mercury toxicity.
Some specific nutrients that have been shown to be important for kidney health and for reducing the effects of mercury toxicity include:
- Vitamin C: Vitamin C is a powerful antioxidant that helps to reduce oxidative stress in the kidneys caused by mercury exposure.
- Vitamin E: Vitamin E is another antioxidant that helps to reduce oxidative stress and inflammation in the kidneys.
- Zinc: Zinc is an essential mineral that is involved in the detoxification of heavy metals, including mercury. Adequate intake of zinc can help to reduce the toxic effects of mercury on the kidneys.
- Selenium: Selenium is a trace mineral that plays a crucial role in the elimination of mercury from the body. Adequate intake of selenium can help to reduce the toxic effects of mercury on the kidneys.
- Omega-3 fatty acids: Omega-3 fatty acids have anti-inflammatory properties and can help to reduce oxidative stress in the kidneys caused by mercury exposure.
Consulting an Integrative or Functional Medicine healthcare professional can be helpful in determining the best nutritional strategies for reducing the effects of mercury toxicity on the kidneys.
The role of the microbiome
The role of the microbiome in mercury toxicity and its effects on the kidneys is an area of active research. The microbiome refers to the community of microorganisms that reside within the human body, including bacteria, viruses, fungi, and other microbes.
Studies have shown that the microbiome can play a role in determining an individual's susceptibility to mercury toxicity and its effects on the kidneys. For example, changes in the composition and diversity of the microbiome have been associated with an increased risk of mercury toxicity and kidney damage.
In addition, the microbiome has been shown to play a role in regulating the detoxification and elimination of mercury from the body. Some studies have suggested that certain strains of bacteria within the microbiome can increase the elimination of mercury while others can reduce it.
Synergy with other environmental toxins
Synergistic exposure to other toxins can play a role in the toxicity of chronic mercury exposure on the kidneys. Synergistic toxicity occurs when exposure to multiple toxins results in a greater combined effect than would be expected from the sum of the individual toxicities of each substance.
In the case of mercury exposure, the presence of other toxins, such as lead, cadmium, arsenic, or pesticides, can increase the toxic effects of mercury on the kidneys. This synergistic effect can result in greater oxidative stress, inflammation, and cellular damage to the kidneys.
Preventing Mercury Toxicity in the Kidneys
To reduce the risk of mercury toxicity in the kidneys, it is important to limit exposure to mercury and to practice good habits. Some ways to reduce exposure to mercury include:
- Decrease intake of high-mercury fish and seafood, such as shark, swordfish, king mackerel, and tilefish.
- Replacing old mercury-containing thermometers and dental fillings with safer alternatives.
- Avoiding contact with mercury-containing products, such as batteries and certain chemicals.
- Washing hands thoroughly after handling any mercury-containing products.
The Bottom line
In conclusion, mercury toxicity is a serious health issue that can cause significant damage to the kidneys. Chronic exposure to mercury can result in various kidney-related health problems, including nephrotoxicity, tubular necrosis, interference with kidney enzymes, and glomerular injury. To reduce the risk of mercury toxicity in the kidneys, it is important to limit exposure to mercury and to practice good lifestyle habits.
January Research and News 2023
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney January Research and News.
Metabolic markers of ultra-processed food and incident CKD
In this research study published in CJASN this month, investigators looked at the link between metabolic markers of ultra-processed food intake and the incidence of chronic kidney disease (CKD).
The evaluated data from 3,751 participants in the Atherosclerosis Risk in Communities (ARIC) study. Dietary intake was assessed using a food frequency questionnaire, and ultra-processed food was classified using the NOVA classification system.
Then they identified the association between 359 metabolites and ultra-processed food consumption. After that they looked at the statistical association between these metabolites and incident CKD.
Higher levels of 3 metabolites mannose, glucose, N2, N2-dimethylguanosine were associated with high risk of incident CKD after a median follow up of 23 years.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Nephrotoxicity of antimicrobials and antibiotics
This review article is a good resource for you to have. It summarizes the various mechanisms for nephrotoxicity of various antibiotics and antimicrobials.
The article also details preventative actions that be used to reduce long-term complications from these antibiotics. These include:
1. Dose adjustment according to kidney function.
2. Appropriate hydration during to course of therapy.
3. Avoiding the use of other nephrotoxins at the same time.
4. The use of as short of therapy as possible.
5. Some reports showed benefit of agents such as vitamin E, vitamin C, N-acetylcysteine, erythropoietin, α-lipoic acid, curcumin, or statins to prevent antimicrobial-induced acute kidney injury.
The full review article is available in the link below. Download it for free and keep it for your future use.
Zinc deficiency link to CKD and hypertensio
This review article published in Kidney 360 discussed the potential link between zinc deficiency, CKD and hypertension.
The article highlights the mechanisms of zinc procurement and trafficking. It provides evidence that urinary zinc wasting can fuel Zn deficiency in CKD. It also discusses how Zn deficiency can accelerate the progression of hypertension and kidney damage in CKD.
Finally, the authors discuss the consideration of zinc supplementation as an exit strategy with the potential to rectify the course of hypertension and CKD progression.
To read the full article please contact us. A link to the abstract is listed below.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Integrative Nephrology: A personalized Approach to Kidney Care
Integrative Medicine combines practices and treatments from alternative medicine with those of conventional medicine. It bridges the gap between lifestyle and functional medicine, and conventional medicine. It is crucial in managing complex chronic diseases such as kidney disease. Here, we will discuss Integrative Nephrology.
Integrative Nephrology
Integrative Nephrology focuses on six primary areas which identify individualized risk factors for kidney disease to develop a personalized lifestyle modification plan that can be incorporated into patient management. The purpose of this publication is to discuss the integrative approach to kidney care in detail.
This is important because chronic kidney disease (CKD) affects millions of Americans and is often associated with comorbid conditions such as cardiovascular disease, diabetes, and hypertension.
According to the USRDS data, almost 15% of the US population has CKD (1 in 7 people), and 1 in 3 are at risk for CKD. Similar statistics are found throughout the world. In addition, nearly 800,000 people suffered from ESKD (end-stage kidney disease) as of December 2018.
Current approach
One in nine patients with kidney disease doesn’t know they have it. The current conventional medicine approach relies on patients to develop signs or symptoms of an illness that is often silent. It tries to identify risk factors such as diabetes and hypertension and depends on busy providers to screen patients for early signs of kidney disease. This delays kidney disease detection and interventions that could reverse it.
Additionally, some kidney diseases are due to autoimmune diseases. Unfortunately, current models of care do not allow providers to identify the predispositions, triggers, and modifiers for these disorders.
Finally, it is thought that less than 10% of kidney diseases are due to genetics. The approach to these overt hereditary diseases has mainly relied on providers observing the progression to ESKD and managing complications.
Most of the tools available to health providers are limited to pharmaceuticals designed to manage diabetes, hypertension, and hyperlipidemia. While these tools are helpful, they tend to encourage the patients to live with their disease instead of attempting to reverse it.
In addition, the current fee-for-service (FFS) model does not allow enough time for the provider to get to know their patients well, identify their risk factors, screen them, and treat them properly. Providers also have to jump through hoops, such as prior authorizations and clunky electronic health record metrics, which do not benefit patient care.
Medicare spent $81 billion on FFS for beneficiaries with CKD in 2018. That is more than 22% of Medicare FFS in that year. This does not include the $49.2 billion that Medicare spent on ESRD services. Yet, despite this massive spending on kidney disease and treatment, there continue to be gaps in identifying people at risk for CKD and preventing and treating CKD.
Integrative Approach to Kidney Care
Integrative Nephrology aims to identify the root cause of kidney disease and sift through multiple factors contributing to the disorder. It mainly focuses on six primary areas or principles which identify individualized risk factors for kidney disease to develop a personalized lifestyle modification plan that can be incorporated into patient management. This approach considers genetics, environment (diet, nutrition, toxins), lifestyle, the gut-kidney axis, and pharmacological interventions.
The six basic principles that Integrative Nephrology uses to assess, address, and treat kidney disease are:
· Genetics and epigenetics
· Nutrition
· Environmental toxin exposure
· Gut-kidney connection
· Lifestyle risk factors
· Pharmaceuticals
Addressing these areas starts with educating patients and providers. Using Integrative and Functional Medicine tools can help develop a personalized lifestyle modification and treatment plan through which the patient can be coached.
Education
Shifting the paradigm from the conventional approach to an integrative requires investing time and effort in educating the patients and raising awareness of this silent and deadly disease.
In addition, it is crucial to educate various providers on the principles of Integrative Nephrology and the tool required for personalized assessment and testing. This often necessitates a team approach.
Personalization
The integrative approach to kidney disease requires a team of trained integrative nephrologists, dietitians/nutritionists, and coaches. It assesses and analyzes all the factors associated with six principles to tailor a “lifestyle modification plan” that can be incorporated into the conventional medicine approach to an individual’s kidney disorder.
Coaching
However, it is not enough to prescribe the patient a lifestyle modification plan. This is because it takes, on average, 66 days for a patient to develop a new habit. Some even need more than six months to establish a new habit. Therefore, patients need guidance in purchasing groceries, preparing meals, stress management, dealing with relationships, managing sleep, etc.
This gap can be filled by coaches trained in the integrative approach to kidney disease. They can guide patients under the supervision of nephrologists and dietitians/nutritionists. A coach can even be a medical assistant who is adequately trained. This approach has been studied and found to improve diabetes and cholesterol control. Coaches have been used in the UK for that purpose. They are integral to the success of our integrative approach to kidney health.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The Principles of Integrative Nephrology
Genetics of kidney disease
The most significant advance in kidney disease in the past few years has been the use of genome-wide association studies (GWAS) in various kidney disorders. In these studies, various kidney diseases were statistically linked to specific variants in the genetic code.
Current evidence suggests that genetics play a role in the development of kidney disease. Common genetic disorders associated with kidney disease include polycystic kidney disease, Alport’s Syndrome, and Fabry’s disease. However, the advances in genome-wide association studies helped identify several hundred other genes linked to kidney diseases. This made genetic testing a valuable tool for identifying CKD risk factors and managing them.
However, genetics is one of the factors that lead to the development of kidney disease. For genes and their variants to contribute to a disease, we need to understand all the factors that explain why people with even the same variant may have different outcomes. Therefore, understanding the interaction between genetics, epigenetics, and the environment is crucial.
In essence, genetics provides the blueprint that may predispose a person to kidney disease, while other factors such as nutrition, lifestyle, and environmental toxin exposure may enhance or counteract that genetic predisposition. Or, as it has been described: genetics loads the gun, and the environment pulls the trigger.
Epigenetics
Epigenetics is a developing field that studies how a person’s behavior and environment can cause changes that affect the way genes work. Researchers found that these factors can cause changes in the DNA, such as histone modifications, methylation, and cytosine hydroxy-methylation. These changes, in turn, can increase or decrease gene expression.
So, just as good lifestyle habits can reduce disease risk by turning off problematic genes, poor decisions can increase our disease risk by turning them on.
Lifestyle factors like diet, sleep, meditation, and exercise, for example, are things we have control over and tremendously influence genetic expression. Limiting exposure to environmental toxins (like food additives, pesticides, heavy metals, and plastics) and supporting the body’s natural detoxification pathways is another essential aspect to keep in mind.
Therefore, identifying genetic predispositions through genetic testing can help providers develop a personalized lifestyle modification plan to promote healthy, beneficial gene expression. This can even be done for genetic disorders like polycystic kidney disease.
Pharmacogenomics
Variability in response to pharmaceutical therapy is due to multiple, complex individual variations in fundamental physiological differences like age, sex, weight, metabolism, absorption, organ function, and disease state. These variables mean patients require monitoring, dose adjustments, and sometimes alternative therapy. Thanks to advances in the field of pharmacogenomics, clinicians can use genetic information to personalize drug therapy. This is the basis of precision prescribing and has a significant benefit in prescribing kidney transplant medications.
Nutrition in CKD patients
Nutrition plays an essential factor in the progression of CKD and outcomes of kidney disease patients. Dietary changes such as low protein and very low protein diet with keto-acid analogue supplementation have been associated with slowing the progression of kidney disease. In addition, a growing body of evidence supports the recommendation for a plant-based or a plant-dominant diet for CKD.
How a nutrient is packaged can also influence its effects on the body. Some forms can be harmful, while others can be beneficial. Dietary phosphorus, for example, when ingested in processed food, can have higher bioavailability and is more dangerous for kidney patients. On the other hand, a diet rich in plant-based whole foods (and rich in organic phosphorus) is recommended for kidney disease. This form of phosphorus is less harmful to the kidney patient.
In general, processed food is devoid of micronutrients and can be high in sodium and phosphorus. It can also potentially contain toxins. Processed food should, therefore, be eliminated for kidney patients.
When considering nutrition for kidney disease, one should also consider food preparation methods, which can lead to the formation of advanced glycation end products (AGEs). AGEs form when sugars react with various foods at high heat, making the food more inflammatory. They have been implicated in diabetes and kidney disease. Cooking methods can also affect the micronutrient content of vegetables.
Furthermore, micronutrients such as zinc and selenium exert many biological functions throughout the body. Zinc deficiency, for example, is well-established in CKD (for more about this, read this blog). In addition, many of the medications that kidney patients use can lead to nutritional deficiencies. Identifying and supplementing these deficiencies can also improve the outcomes of CKD patients. For more about this, read here.
Finally, realizing that nutrients don’t work in isolation is crucial. Biological functions are more complex and involve multiple nutrients and vitamins as cofactors. These complex interactions may explain conventional research's failure to identify the link between a single nutrient and various outcomes.
Vitamin D, for example, interacts with vitamin K2 and magnesium to optimize bone and vascular health. Likewise, b vitamins are given as a B-complex because of the interactions between multiple B vitamins in biochemical pathways.
Individualizing a nutritional “prescription” for a specific patient, therefore, requires diligent evaluation of nutritional status, drug-induced nutritional deficiencies, nutrient interactions, and nutrigenomics.
Supplements under nephrologist supervision
Traditionally, nephrologists have been opposed to providing supplements to kidney disease patients. This is supported by case reports of supplement-induced acute kidney injury. In addition, there is no doubt that the supplement industry is poorly regulated. However, as mentioned above, many kidney disease patients suffer from nutrient and micronutrient deficiencies. Therefore, an individualized and comprehensive assessment of nutritional status is necessary to optimize care for these patients. Supplementing these deficiencies under the supervision of nephrologists using high-quality supplements can fill this gap. You can read this well-written blog on how to choose a high-quality supplement.
Environmental toxin exposure
Each year, 220 billion tons of toxic chemicals are released into the environment. In addition to the liver, the kidneys are essential for the biotransformation (the breakdown) of many of these chemicals. They are also the primary route of elimination for many. Furthermore, some substances selectively accumulate in the kidneys and cause chronic kidney disease or even kidney cancer.
Environmental toxins, therefore, should be assessed and addressed in a comprehensive plan for kidney health. For example, polyfluoroalkyl substances (PFAS) are a family of greaseproof, waterproof, and non-stick industrial compounds. They are used in hundreds of consumer products, including ones touching your food and skin. You might have heard of them as “forever chemicals” or Persistent Organic Pollutants (POPs). Researchers have shown that they can lead to kidney damage.
Even chemicals thought to be problematic only in the past are becoming a current environmental issue. For example, toxins such as arsenic, lead, and cadmium are found in processed beverages, drinking water, and cigarettes. All three have been implicated in the development and progression of kidney disease. In fact, lead is one of the major risk factors for kidney disease worldwide.
Decreasing exposure to these chemicals and others is essential in preventing kidney disease. Understanding the genetics involved in metabolizing and excreting these toxins can help individualize their management.
In addition, improving the body’s ability to bio-transform them can slow down the progression of kidney disease. This can be accomplished by a personalized nutritional evaluation and lifestyle modification program. Pharmaceuticals can even be used to clear high levels of toxins when indicated.
The gut-kidney connection
Dysbiosis and intestinal permeability are implicated in many systemic inflammatory and immune-related disorders that lead to chronic disease. Dysbiosis describes unfavorable alterations in the gut microbiota, including the overgrowth of harmful bacteria or poor growth of good bacteria. The normal integrity of the gut can be compromised by dysbiosis and cause systemic complaints, even in the absence of overt gut symptoms.
The gut-kidney connection refers to the relationship between gut integrity, microbiome diversity, and kidney disease. The connection between the gut and kidneys is very complex. Still, we can break it down into two major categories: the gut-derived uremic toxins and the inflammatory immune response that can trigger kidney disease.
Research has established a relationship between gut microbes, gut integrity, and kidney disease. This occurs through very complex biochemical and immune mechanisms. The presence of "good" bacteria has been associated with immune changes that inhibit inflammation. The use of probiotics and prebiotics that produce favorable changes in the microbiome has also been associated with gut changes that reduce many uremic toxins associated with advancing kidney damage.
Comprehensive gut assessment and restoration protocol
Conventional medicine and research realize the importance of the gut microbiome. Its literature is even paying increasing attention to gut integrity. However, the approach to studying various interventions focuses on using “the scientific method” to isolate a single modifier, such as probiotics or symbiotics, and assess its benefit. In doing so, they usually fail to address other aspects of gut restoration and mucosal repair that may be required to achieve desired outcomes.
A more comprehensive and individualized approach is the so-called 5R protocol. In this protocol, providers help patients remove potential triggers to gut hyperpermeability, replace nutrients and digestive aids that are deficient, reinoculate good bacteria when needed, repair gut integrity and motility, and rebalance lifestyle factors for a healthier gut.
Lifestyle risk factors
In nearly all guidelines, lifestyle modification is the first step recommended for managing chronic diseases. Yet, guidelines often fall short of describing what lifestyle modifications patients should be applied and how. It is often thought that lifestyle modifications are limited to diet and exercise. Smoking and alcohol consumption are sometimes added to the mix.
There is no doubt that diet and exercise are essential. In fact, diet is so important that we made it an independent principle of our integrative approach to treating kidney disease. However, lifestyle modifications go beyond that to managing stress, sleep, and having healthy relationships.
Many CKD patients live stressful lives. They may have stressful jobs, toxic relationships, and underrecognized sleep abnormalities. All these lifestyle problems, in addition to diet, exercise, and smoking cessation, can influence the progression of kidney disease.
Managing stress through breathing exercises, meditation, and yoga can have a tremendous impact on blood pressure and diabetes control and can even slow the progression of kidney disease.
In addition, healthy relationships have been associated with better cardiovascular outcomes. Giving and receiving support, affection, help, and advice can trigger the release of stress-reducing hormones. Social relationships can relieve stress. Lowering stress positively affects heart health, insulin regulation, and the immune system and strongly benefits kidney health.
Pharmaceuticals
Nothing in our integrative approach to kidney health is against using medications to manage kidney disease. However, studies showed that an average kidney disease patient takes nine medications. Our integrative approach utilizes medicines as a last resort. In some instances, medications are necessary while simultaneously addressing the other basic principles (genetics, nutrition, etc.) to prevent rapid loss of kidney function.
For example, if a patient is experiencing vasculitis (inflammation of the blood vessels), managing and decreasing inflammation using immunosuppressants may be essential to avoid the rapid loss of kidney function. This can be done parallel with a “root cause” workup that investigates environmental exposures, gut-kidney factors, other lifestyle choices, and events that could have triggered or perpetuated the vasculitis.
Furthermore, when using medications for kidney disease, one must pay special attention to a few unique issues. First, the dosing of medicines often requires changes based on kidney function. The kidneys excrete many medications or their metabolites, and adjusting their doses is essential to avoid toxicity.
Second, providers need to pay attention to drug-drug interactions that are more important in kidney patients due to the higher number of medications involved. Third, what should be noticed by many providers is the drug-nutrient interactions. Many medications used for kidney disease are associated with nutrient deficiencies that can affect outcomes. For example, the use of ACE inhibitors, which is common in kidney patients, can be associated with zinc deficiency.
Tools of Integrative Kidney Care
While conventional medicine utilizes simple, tried, and true tools for evaluating and managing kidney disease, Integrative medicine uses tools that dig deep to identify the patient's individual characteristics to help develop a personalized plan.
Conventional medicine, for example, uses tools such as basic metabolic panels, renal panels, urinalysis, urine albumin to creatinine ratio, urine protein to creatinine ratio and serologic testing, and even kidney biopsies. However, while these tests may help identify the type of kidney disease the patient is suffering from, they do not detect the root cause nor help the patient and provider navigate through a personalized approach to rid the patient of that disorder.
Integrative Nephrology utilizes tools such as genetic testing, environmental toxin exposure evaluation and testing, nutritional evaluation and testing, evaluation of gut integrity and microbiota, and lifestyle assessments.
Genetic testing
Testing for evaluation
Various methods for identifying genetic variants associated with kidney disease have been used. The exome is that 2% of the genome that codes for all biological proteins. Next-generation sequencing is a new technology that allows DNA sequencing of the entire human exome within a single day. It also captures a broader spectrum of variations that can affect the genetic code.
This revolutionary technology can identify mutations associated with CKD. It can also recognize variants associated with an increase in the risk or severity of CKD. It is now available for kidney disease patients (Natera's Renasight test). This “broad-panel genetic testing” type can transform care for patients with kidney disease.
Testing for management
Many companies also provide pharmacogenomic testing to guide medication prescribing to avoid toxicity and side effects. One of these companies is Genetworx which provides a comprehensive report. Even Quest Diagnostics offers pharmacogenomic testing.
Nutrigenomic testing is also helpful in guiding nutritional evaluation and management for many kidney patients who tend to grapple between nutritional deficiencies and excesses. Unfortunately, the field of nutrigenomics is still in its infancy. While companies such as Nutrition Genome and Nutrigenomix can be used for that purpose, these tests may still need to be standardized.
Nutritional assessment and testing
A comprehensive nutritional assessment includes anthropometric measurements of body composition, serum protein, micronutrients, and metabolic parameters, clinical evaluation of altered nutritional requirements and social or psychological issues that may preclude adequate intake, and dietary intake. A collaboration between an Integrative provider and a dietitian or nutritionist is essential for this assessment.
Techniques for measuring body composition of fat and lean body mass include anthropometry and bioelectric impedance analysis. Devices such as bodystat or inbody can be used for that purpose.
A 3 day-diet recall can be used to identify dietary intake of various nutrients. In addition, multiple tests are commercially available to assess different nutrients and metabolic parameters. Genova’s NutrEval is one of our favorites.
Environmental exposure assessment and testing
Various surveys have been developed for assessing environmental toxins exposure. The CDC has a comprehensive questionnaire that can be used for that purpose. The Institute of Functional Medicine also developed a survey that can be downloaded here.
Unfortunately, after chronic exposure to heavy metals and forever chemicals, our bodies adapt and store them in tissues such as fat or bone. So, a simple blood test may not detect these substances. The best analysis to detect them is a provoked urine collection. This requires your physician to order a medication that helps release these substances from their storage.
However, a simple analysis is unprovoked timed 6-hour urine collection can be a starting point. We like Doctor’s Data Urine Toxic and Essential Elements test for that purpose.
Gut integrity and microbiota testing
Gut health questionnaires can be used as a starting point during patient intake. The World Gastroenterology Organization, for example, has a patient questionnaire that can be useful.
However, it is essential to realize that more than these questionnaires may be required, and patients could have dysbiosis and increased intestinal permeability without gut symptoms.
This is why we recommend using stool testing for dysbiosis and intestinal permeability. We like Doctor’s Data GI 360 stool tests and Diagnostic Solutions GI map test (the latter is more comprehensive).
Lifestyle assessment
Assessment for lifestyle factors usually involves a comprehensive intake form that asks the patient about various rituals related to sleep, relaxation, exercise, and relationships. We recommend updating these assessments at least annually. You can download our comprehensive intake form here.
Barriers to an integrative approach to kidney disease
To implement an integrative approach to kidney health and disease, providers need more time to get to know their patients, identify their struggles and challenges, and guide them. This is crucial to tailor a lifestyle modification plan individualized to the patient and the specific kidney disorder.
In addition, it requires adequate provider, dietitian, and nutritionist integrative training. The availability of trained integrative coaches is also crucial for this approach to succeed.
Many providers have used the direct patient care model to implement an integrative or functional medicine approach to chronic diseases. However, this approach is disadvantageous to many kidney disease patients, many of whom are in more vulnerable populations.
Therefore, it is critical to implement changes in the healthcare reimbursement model that allow for more time with patients and reimbursement for nutrition, lifestyle management, and coaching. This approach could save costs in the long run and provide more years of kidney health and better quality of life for patients.
The Bottom Line
Our integrative approach to kidney disease focuses on identifying individualized risk factors for kidney disease to develop a personalized lifestyle modification plan that can be incorporated into the patient’s medical management. The patient will then be coached to help them succeed in achieving these modifications and build new healthier habits. The current reimbursement model needs to change to accommodate this approach which could reduce costs and improve kidney patients' outcomes.
November Research and News
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney November Research and News.
Be aware of Rosuvastatin
In this real-world study, investigators analyzed electronic health records of 152,101 and 795,799 new users of rosuvastatin and atorvastatin, respectively, from 2011 to 2019.
The analysis identified 2.9% of patients with hematuria and 1.0% with proteinuria during a median follow-up of 3.1 years.
Compared with atorvastatin, rosuvastatin was associated with an increased risk of hematuria, proteinuria, and kidney failure requiring renal replacement (dialysis or transplant).
44% of patients with severe CKD were prescribed a higher dose of rosuvastatin than recommended by the FDA.
The maximum daily dose of Rosuvastatin should not exceed 10 mg for patients with severe CKD (eGFR <30 ml/min per 1.73 m2).
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Toxic Occupational Exposures and Membranous Nephropathy
In this observational epidemiologic study, researchers compared the occupations and toxic occupational exposures of 100 patients with membranous nephropathy with those of the general population, consisting of two cohorts of 26,734,000 and 26,500 French workers.
Patients with membranous nephropathy worked more frequently in the construction sector than the general population. This difference remained significant by age and sex.
They were also more frequently exposed to toxic substances, such as asbestos, lead, or organic solvents, than the general population.
When thinking about glomerulonephritis one should think about predispositions, triggers, and mediators leading to inflammation.
This study may indicate that toxic occupational exposures may be one of the triggers for membranous nephropathy.
Higher baseline vitamin D levels are associated with a lower risk of CKD in patients with diabetes
Investigators analyzed serum 25OHD levels in 348,243 adults from the UK Biobank without prior CKD at baseline. They looked at the risk of new-onset CKD in diabetic and non-diabetic patients.
During a median follow-up duration of 12.1 years, 9,344 new-onset CKD were documented.
Among participants with diabetes, 25OHD ≥50 nmol/L was associated with a significantly lower risk of new-onset CKD than those with baseline serum 25OHD <25 nmol/L in diabetic patients only.
It is, therefore, not enough to target a "normal" lab value of 25OHD of 30 nmol/L. Providers should aim for levels higher than 50 nmol/L.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Grilled Chicken Greek Salad
In this new category, we will post once a month practical tips and tricks for healthy living with kidney disease. Consider these as tools that you can modify according to your unique situation. These tools span across our principles of Integrative medicine approach for kidney disease, including genetics, environmental exposure, gut health, lifestyle, and others. We invite others to contribute to this category. If you are interested in contributing, please contact us info@inkidney.com. In this first blog, we will share a recipe for making a grilled chicken greek salad.
Plant-dominant diets have been shown to delay the progression of kidney disease. With proper implementation, eating plant-dominant provides a good source of alkali-promoting foods without significant risk of raising potassium or phosphorus levels.
Ideally, a plant-based diet should include 8-10 servings per day primarily of vegetables, with some fruits. For any given meal, vegetables and fruits should be the main dish with animal protein, if included, as a side dish, not the main attraction. This is often the opposite of how many people typically center on meat or poultry as the main event, with vegetables serving as sides. Fruit is also recommended, choosing those lower in carbohydrates, and higher in fiber and phytonutrient benefits.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Grilled Chicken Greek Salad
This recipe is a healthy plant-dominant lunch with 22 g of protein and 10 grams of fiber. It is a good option for lunch and can be prepared ahead of time.
Ingredients
The dressing
- 1/4 cup of organic olive oil
- 1/4 cup of lemon juice
- cracked pepper to taste
- 2 tablespoons of dried oregano
- 1 tablespoon of red wine vinegar
- 2 fresh large garlic cloves, minced
- All-purpose Greek Seasoning to taste
The salad
- 4 oz of organic boneless chicken breast
- 1 cup of romaine lettuce leaves (washed and shredded)
- 1/4 large cucumber, sliced
- 1/4 red onion, thinly sliced
- 2 oz of fresh feta cheese, cubed
- 4 organic grape tomatoes, cut in halves (half of that if you are on potassium restrictions)
- 1/4 avocado (skip if you are on potassium restrictions)
- 1/4 cup of pitted organic kalamata olives, halved lengthways
- 1/4 organic medium green pepper deseeded and sliced
- 1 tablespoon of organic lightly salted pumpkin seeds
Directions:
- Whisk together all the dressing ingredients in a shallow dish. Pour 1/4 cup to use as dressing. Reserve the rest in the refrigerator for later use.
- If desired, you can marinate the chicken in this dressing for 30 minutes before grilling. While the chicken is marinating, prepare all the salad ingredients (except for the avocado) in a large bowl and mix.
- Heat a large grill pan or cast-iron skillet over medium-high heat. Remove the chicken from the marinade and add to the pan. Grill or sear until the chicken is golden on the outside and cooked through (5-6 minutes on each side).
- Remove chicken from the pan and allow to rest for 5 minutes. Slice and arrange over salad.
- Slice avocados and arrange on salad.
- Add salad dressing.
- Garnish with pumpkin seed.
Enjoy!
While the publisher and author have used their best efforts in preparing this blog, they make no representations or warranties with respect to the accuracy or completeness of the contents of this blog and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for you or your patients. You should always consult with a professional where appropriate. Neither the publisher nor the author shall be liable for any loss of profit or any other personal/commercial damages, including but not limited to special, incidental, consequential, or other damages.
October Research and News
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney October Research and News.
Escherichia- Shigella dysbiosis is common in IgA nephropathy patients
This study conducted by investigators in Xijing Hospital, China examined 127 patients with IgA nephropathy (IgAN) who were treatment-naive and 127 matched healthy controls. They also studied 40 patients with membranous nephropathy.
Fecal microbiota samples were collected from all participants and analyzed using 16S ribosomal RNA sequencing.
Expansion of the taxonomic chain Proteobacteria–Gammaproteobacteria–Enterobacteriales–Enterobacteriaceae–Escherichia-Shigella was observed in patients with IgAN who were treatment-naive.
Escherichia-Shigella contributed the most and was determined to be the optimal bacterial classifier of IgAN.
Gut dysbiosis in predisposed patients may be a trigger in a chain reaction that leads to IgAN in some cases. Correcting it may help reverse this process. However, every patient is different and these types of studies should not be interpreted as the sole association with IgAN. Because the trigger of IgAN can vary from patient to patient.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Air pollution may increase the risk of kidney failure in patients with IgA nephropathy
Since we are discussing IgAN, let's discuss this new study that was published in Kidney International. Here investigators studied 1979 patients with IgAN from different provinces in China.
PM2.5 exposure from 1998 to 2016 was derived from satellite aerosol optical depth data. The severity of PM2.5 exposure in different regions was correlated with regional kidney failure burden.
Each 10 μg/m3 increase in the annual average concentration of PM2.5 exposure before or after the study entry was associated with an increased risk for kidney failure after adjusting for other variables.
When thinking about autoimmune kidney diseases (glomerulonephritis) from an Integrative medicine approach, we think about predispositions, triggers, mediators, and inflammation.
Air pollution, in this study, is proven to be a mediator in the progression of IgAN.
Plasma cadmium levels are associated with kidney stone
This case-control study assessed 940 patients with confirmed kidney stones and controls. Investigators measured plasma heavy metals.
They found a correlation between plasma cadmium levels and kidney stones. This association remained strong after adjusting for other confounders.
Exposure to cadmium mainly occurs through ingesting contaminated food, water, or inhalation. The most significant sources of cadmium exposure include batteries, pigments and coatings, plastics, phosphate fertilizers, and tobacco cigarettes.
Cadmium is associated with many adverse outcomes such as cancer and organ toxicity. It has been associated with kidney injury as we discussed in this blog.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Proton Pump Inhibitors Wean Protocol
Proton pump inhibitors (PPIs) are a group of medications that suppress stomach acid production. They work by suppressing the pump that produces acid in the stomach lining. These medications can be very effective and have a significant role in treating stomach ulcers in the acute setting. However, the long-term use of PPIs can be associated with various adverse outcomes. Observational studies demonstrated a link between using proton pump inhibitors and kidney disease. Here we will describe our favorite proton pump inhibitors wean protocol.
By Majd Isreb, MD, FACP, FASN, IFMCP
PPIs use is so common. I am confronted with patients on long-term PPIs every day in my practice. The indication ranges from acid reflux to a history of gastrointestinal bleeding. These medications are now available over the counter and relatively inexpensive.
Patients taking PPIs for a long time without a specific indication should be reassessed. However, gradual and careful de-prescribing should be considered for these patients under an experienced Integrative medicine provider.
Proton pump inhibitors wean Protocol
Remove triggers
- Foods, especially acidic: spicy, fatty foods, alcohol, caffeine, and dairy. Consider an elimination diet.
- Increased intra-abdominal pressure: Encourage weight loss and avoid tight-fitting clothes.
- Stomach over-distension: Encourage smaller meals and less fluid intake with meals. Slow down, chew food well and relax while eating.
- Prone position: Eat last meal 4-5 hours before bed, and place 4-6” blocks under the head of the bed (don’t prop on pillows as this can increase intra-abdominal pressure).
- Smoking: Stop.
- Stress: See “Rebalance” below.
Replace
- Vitamin/mineral deficiencies: Consider B12, magnesium, calcium, and iron. (Goal for B12 > 400 pg/mL. Measure RBC Mg, not serum Mg).
- More fruit and vegetables and fiber.
- Digestive enzymes if needed.
- Consider Swedish bitter.
- Consider MMC Restore from Gaia herbs.
Repopulate
- Consider testing for SIBO.
- Raw apple cider vinegar or other fermented food (sauerkraut) as tolerated.
- If signs/symptoms of small bowel bacterial overgrowth (bloating, gas, diarrhea, abdominal cramps) from poor digestion, consider probiotics (10-14 billion units daily, multiple species present).
Repair
- Add one or more of the following:
- Marshmallow Root Tea: up to 5-6 g daily or 5 mL tincture before meals.
- DGL: 1 chewable tablet (300-500 mg) thrice daily.
- Slippery elm: 1-2 tbs powdered root in water OR 500 mg caps OR 5 mL tincture three times a day.
- Chamomile: 1-3 g in tea, three to four times daily.
- Melatonin regulates gastric acidity by inhibiting excess acid formation and increases the tone of the lower esophageal sphincter, which makes sense. The body wants to keep acid in the stomach when lying down to sleep. Start with 1 to 3 mg daily one hour before going to bed.
- Throat Coat tea: Can be taken with meals (it contains regular licorice and may raise blood pressure).
Rebalance
- Decrease stress: Lifestyle changes, mind-body techniques, vagus nerve stimulation.
- Regular aerobic activity: but not right after meals.
- Consider other modalities: such as acupuncture weekly for 2-4 weeks.
Taper off the PPI slowly.
- The higher the dose, the longer the taper. Expect rebound symptoms.
- Start supplements as above two weeks before taper.
- Decrease the current PPI dose by 50% each week until the patient is on the lowest dose once daily.
- In 2 weeks, change to an H2 blocker (Pepcid, for example). If symptoms flare, you can alternate H2B every other day with PPI.
- After 2-4 weeks on H2 blocker, try stopping or weaning.
- After two weeks off the H2 blocker, try tapering off supplements.
- Continue lifestyle modifications.
If you are taking a PPI, please do not stop them suddenly, as this can lead to a severe rebound. Please consult with your provider before discontinuing a PPI. We highly recommend tapering PPI slowly under the supervision of a well-trained provider.
Occupation and kidney stones
Kidney stones are often painful and, if left unaddressed, can lead to more severe conditions such as obstruction of urinary flow and permanent kidney damage. Recurrent kidney stones are one of the common causes of CKD. Kidney stone development is the composite result of multiple factors, including genetic, dietary, and electrolyte imbalances and environmental factors. But is there a relation between occupation and kidney stones? Here, we will discuss the role of work in kidney stone formation.
By Majd Isreb, MD, FACP, FASN, IFMCP
Calcium oxalate stones are the most common type of kidney stone. It is often thought that it is due to multiple factors, including genetics, high sodium intake, diminished water intake, and increased animal protein and dysbiosis. This compilation leads to the formation of acidic urine supersaturated with calcium and oxalate. But does your type of work increase your risk for kidney stone formation?
Occupation and kidney stones
When you think about the type of jobs associated with increased risk for kidney stone formation, it is essential to consider the factors associated with increased risk of kidney stones and how they are affected by various jobs.
Therefore, it is crucial to understand the patient's job and how it affects their access to water and bathroom facilities. It is also essential to consider exposure to high ambient temperatures and chemicals.
Meet Candice!
Candice (not her real name) is a patient with a history of multiple calcium oxalate kidney stones. Despite numerous attempts at improving her dietary intake, oxalate, and measures to decrease urinary calcium and oxalate supersaturation, she continued to have kidney stones.
Candice works as a visiting nurse for a home health agency. With ever-growing demands to see more patients, she has not been able to drink the necessary amount of water to decrease her risk for kidney stones. She feels uncomfortable going to her patients' homes and using their bathrooms.
Lack of access to filtered water and bathroom facilities plays a vital role in increasing the urinary concentration of calcium and oxalate (and other minerals). These types of jobs increase the risk of forming kidney stones.
In fact, this study found that healthcare professionals are at higher risk of kidney stones. The risk was exceptionally high for surgeons in the operating rooms.
Jobs in higher temperatures
Exposure to higher temperatures has been linked higher risk for kidney stone formation. Temperature higher than 86oFincreases the risk of kidney stones after less than three days. Physical jobs that require spending a significant amount of time outdoors in warmer climates can be associated with increased perspiration and more concentrated urine. This can raise the risk of kidney stone formation.
Sedentary jobs
Those who work indoors in an office environment are not safe either. The link between a sedentary lifestyle and metabolic syndrome is well established. Since metabolic syndrome is associated with increased urinary calcium, uric acid, oxalate, and decreased urinary citrate, it has been linked to an increased risk of kidney stones.
Office jobs that involve lots of sitting can aggravate metabolic syndrome, increasing the risk of kidney stones. These jobs include computer technology, administrative options, communication, and transportation, for example.
What about astronauts?
According to NASA, astronauts have reported postflight kidney stones more than 30 times. There is a reason why astronauts are at increased risk of kidney stone formation. Living and working in a zero-gravity environment accelerate bone loss and muscle atrophy. This has been found to increase urinary calcium and calcium oxalate supersaturation.
In addition, many astronauts may not drink enough water while in space. This leads to further increased calcium and oxalate excretion. Finally, astronauts consume a higher amount of processed food containing higher sodium which increases the risk of kidney stones.
Exposure to oxalic acid at work
Oxalic acid is a colorless, odorless powder. It is used as a rust remover, radiator cleaner, and ink stain remover. Oxalate, the ionized form of oxalic acid, is a simple chemical compound that humans cannot break down. Therefore, it is a waste product that the body must eliminate. The kidneys are the primary site of that elimination.
Higher oxalate exposure is toxic, especially to the kidneys. Calcium oxalate stones are the most common type of kidney stones. Increased occurrence of kidney stones was identified in railroad workers who were exposed to oxalic acid in Scandinavia.
Join us to end the kidney disease epidemic
The Integrative management of kidney stones is complex and requires collaboration between an experienced Integrative nephrologist and trained dietitians or nutritionists. Patients in high-risk occupations should strive to increase filtered water intake, when possible, to about 3 liters a day (depending on size). They should also improve their physical activity, limit exposure to toxic chemicals and get frequent bathroom breaks. Those who have demanding jobs that don’t allow them to drink enough may benefit from taking potassium citrate supplementation three times a day.
The bottom line
There is a significant link between occupation and kidney stones. Various jobs can increase the individual risk of kidney stone formation. This can add another layer to the integrative medicine approach to kidney stone prevention.
Effects of Glyphosate on kidney health
Glyphosate is a non-selective herbicide. This means that it can kill almost any plant that it contacts. It is now commonly used in herbicides that control weeds that grow among food crops, gardens, lawns, golf courses, public parks, and walkways. Here, we will discuss the available literature on the effects of glyphosate on kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Glyphosate was patented in 1961 as a chelating agent for mineral deposits that accumulate on pipes and boilers in hot water systems. In 1968, Monsanto (now owned by the pharmaceutical company Bayer) patented glyphosate as an herbicide for agricultural use. It was even patented later as an oral antibiotic.
Because glyphosate is a non-selective herbicide, it was damaging crops. This led to the development of genetically modified organisms (GMO) which are crops resistant to glyphosate. It is estimated that glyphosate is sprayed on U.S. crops at about 150,000 tons yearly.
Humans are exposed to glyphosate by eating crops raised in contaminated soil, drinking contaminated water, and inhaling it. A recent study of the NHANES database found that 80% of American adults and children have glyphosate in their urine.
It has been potentially linked to cancer, mental health issues, endocrine disruption, and other health concerns. But what about its effects on the kidneys?
Glyphosate is present in herbicides as a salt. It is mostly negatively charged, so it binds to positively charged minerals to form various salts. Because of that, it can also bind to other nutrients and heavy metals. This can lead to the depletion of some minerals in crops and increase exposure to heavy metals.
Effects of glyphosate on kidney health
Acute exposure
There have been few case reports of suicide attempts by ingesting glyphosate. These cases were characterized by acute kidney failure due to acute interstitial nephritis, metabolic acidosis, hyperkalemia, and pulmonary edema. Patients may require hemodialysis. Ultimately, they may recover but with persistent chronic kidney disease.
Chronic low-level exposure
The cause of chronic kidney disease of unknown etiology (CKDu) has been debated for years. A recent study showed a possible association between agrochemicals and the development of CKDu.
In 2019, the American Association for the Advancement of Science awarded two Sri Lankan scientists. The scientists reported that glyphosate plays a crucial role in transporting heavy metals to the kidneys of those drinking contaminated water, leading to high rates of chronic kidney disease in farming communities in Sri Lanka.
While the use and exposure to glyphosate are common in the U.S., there is no medical literature connecting it to kidney injury here. Yet, there are various ways that chronic exposure to glyphosate can lead to kidney injury or worsening kidney disease.
Join us to end the kidney disease epidemic
Glyphosate disrupts the mitochondria
The kidneys utilize a significant amount of energy to perform various functions. Many of these functions take place in the tubules. Most of this energy is produced in the mitochondria, which are the powerhouse of the cells.
Glyphosate has been found to cause mitochondrial dysfunction and increase oxidative stress. The mechanism of this complex. However, it is thought to occur by disrupting the mitochondrial membrane potential and oxidative phosphorylation.
Glyphosate also leads to the generation of reactive oxygen species and the depletion of glutathione (the master detoxifier). It also depletes manganese which is necessary for the action of the antioxidant enzyme superoxide dismutase.
Glyphosate disrupts the gut microbiome
Glyphosate was patented as an antimicrobial agent in 2010. It was proven to inhibit an enzyme called ESPS synthase in certain bacteria. However, it turns out that most of the good gut bacteria contain that enzyme. In animal studies, exposure to glyphosate decreased these good bacteria in the gut. Other pathogenic (harmful) bacteria have ways to break down glyphosate, making it less effective (more on this later). This can lead to the growth of these harmful bacteria.
This glyphosate-induced dysbiosis has been associated with immune system disruption. This can potentially worsen inflammation in kidney disease and lead to faster progression of CKD.
The makers of the glyphosate herbicide claim that humans cannot break it down and that it is excreted unchanged in the urine. This claim is debated. It is more established that dysbiotic bacteria can break down glyphosate.
There are two major pathways involved in the way bad bacteria break down glyphosate:
- One pathway breaks it down into phosphate and sarcosine. The latter is further used to produce the amino acid glycine. Phosphate is used for energy production.
- The other process breaks it down into glyoxalate and aminomethylphosphonic acid (AMPA). AMPA is further broken down to formaldehyde. Glyoxalate can further be metabolized to produce glycine and oxalate. Some of that glyoxalate and phosphate may get absorbed.
You can see that this creates a positive feedback loop, in which long-term exposure to glyphosate continuously kills the good bacteria and feeds the harmful bacteria.
Glyphosate and toxic metals
The negatively charged glyphosate can bind to heavy metals. These glyphosate-metal complexes have been proposed as the cause of the epidemic of kidney disease in Sri Lanka. These complexes tend to concentrate in the kidneys and lead to kidney disease.
On the other hand, glyphosate structure is similar to the amino acid glycine. Glycine, like other amino acids, is used to produce protein in the body. Glyphosate substitution for glycine during protein synthesis is thought to be a contributory cause of diabetes, obesity, Alzheimer’s disease, and other modern chronic diseases.
Glycine substitution in the proteins of the aquaporin, chloride channels, cytochrome C oxidase, and collagen has been proposed to contribute to dehydration, increased urinary acidification, kidney scarring, muscle breakdown, and mitochondrial dysfunction.
Glyphosate and acid load
Potential Renal Acid Load (PRAL) measures the amount of acid or alkali the kidneys are exposed to. It is usually used to assess the potential acid load contained in various food. A higher dietary acid load has been linked to the faster progression of CKD.
Chronic exposure to glyphosate may increase the acid load by various mechanisms. As seen above, the dysbiotic bacteria in the gut can continuously break it down with chronic exposure. Formaldehyde is one of the products of this metabolism. It can be further broken down into formic acid.
In addition, glyphosate uncouples oxidative phosphorylation in the mitochondria. This shifts the mitochondrial energy production to perform anaerobic respiration, which generates more lactic acid.
Glyphosate salts ingestion by itself leads to the release of protons (H+) under typical blood acidity. This further increases the acid load on the kidneys.
Finally, some authorities claim that glyphosate can inhibit an enzyme called glutamine synthetase. This is evidenced by the accumulation of glutamate in the nervous system. It is, therefore, plausible that glyphosate can also inhibit the glutamine-glutamate loop between the liver and the kidneys. This can decrease the kidney's ability to handle acid excretion.
Join us to end the kidney disease epidemic
Glyphosate is a source of oxalate and phosphate
As seen above, glyphosate gets metabolized by harmful bacteria in the gut with further production of oxalate and phosphate. While most of the phosphate is used by these bacteria, some of it could get absorbed. This could partly explain the high phosphate burden in the standard American diet (in addition to food additives).
Most importantly, though, oxalate is produced by the glyphosate metabolism. In the setting of the standard American diet, oxalate formed by the dysbiotic gut bacteria can get absorbed. This further increase the oxalate burden on the body. Oxalate is well known to accelerate kidney disease and bind with calcium in the urine to form kidney stones.
What can you do?
So, you can see that glyphosate is all around us, and most of the U.S. population is exposed to it. Bayer said in 2021 that it would stop selling glyphosate-based weedkillers in the U.S. residential market for non-professional gardeners. Yet, it will continue to sell glyphosate-based weedkillers to farmers, who rely on it heavily. Therefore, your crop will likely continue to be contaminated by it.
Whether Bayer eventually stops selling glyphosate completely or not, you need to take steps to protect yourself. Here are a few recommendations:
- Avoid GMO food. Non-GMO certification programs test products to ensure they contain an insignificant amount of GMO material.
- Consume organic produce as much as possible. I understand that sometimes buying all organic can be expensive. So, you can use the EWG Dirty DozenTM list to avoid buying highly contaminated produce. These products must be purchased organic.
- Filter your water using a reliable water filtration system that removes glyphosate. One product that we recommend is ClearlyFiltered®.
- Avoid processed food as much as possible. Processed food is laden with toxins and full of unhealthy preservatives. Soy and corn products in the U.S. are highly processed, and most crops are GMO.
- Enhance your diet with antioxidants and supplements containing glutathione and other nutrients chelated by glyphosate, such as manganese.
The bottom line
Glyphosate is all around us. While no clear epidemiological data link it to kidney disease in the U.S., several mechanisms can lead to potential kidney damage. Taking the steps outlined above can help you decrease exposure.
September Research and News
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney September Research and News.
A pro-inflammatory diet is linked to faster progression of CKD
In a study published ahead of print, investigators analyzed the NHANES data to assess the link between a "pro-inflammatory diet" and the progression of CKD.
1,084 adults with CKD were included. 109 (10%) developed kidney failure requiring renal replacement therapy (dialysis or transplant).
An adapted Dietary Inflammatory Index (ADII) score was calculated to determine the inflammatory score based on dietary recalls.
In addition, an inflammatory score was used. This was based on four markers: CRP, serum albumin, white blood cell count, and mean platelet volume.
Both dietary inflammatory index and inflammatory score were associated with increased progression to kidney failure.
The authors emphasized that dietary saturated fatty acids, trans fats, or sugar are pro-inflammatory and associated with faster progression of CKD. While a diet rich in fiber or n-3 polyunsaturated fatty acids (n-3 PUFAs) are anti-inflammatory effect and is associated with slower kidney function decline.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Synbiotic treatment in kidney disease decrease inflammation
In a small study published ahead of print in the Journal of Renal Nutrition, researchers studied 34 adults with CKD.
Patients were randomized to either receive synbiotic treatment (Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium lactis, 32 billion colony-forming units per day plus 3.2 g of inulin) or a placebo for 12 weeks.
Synbiotic treatment significantly modified the gut microbiome. It reduced serum levels of the gut-derived uremic toxin indoxyl sulfate. It also decreased levels of high-sensitive C-reactive protein. There was a small improvement in the estimated glomerular filtration rate.
These studies are promising. However, a personalized approach based on a comprehensive gut restoration protocol may be more effective in decreasing inflammation.
Gender plays a role in age-related loss of kidney function
In a study published last month in JASN, the authors studied 1837 persons (53% women, aged 50–62 years) without self-reported diabetes, CKD, or cardiovascular disease over a period of 13 years in northern Europe.
GFR was measured by plasma iohexol clearance. Women had a lower GFR than men at baseline. However, the rate of decline in GFR was faster in men than in women.
The relationship between GFR and age was linear in women but was curvilinear in men with a faster decline at an older age. This decline was independent of health status.
This study indicates the potential role of sex hormones in the decline of kidney function and kidney disease.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Proton Pump Inhibitors and Kidney Disease
Proton pump inhibitors (PPIs) are a group of medications that suppress stomach acid production. They work by suppressing the pump that produces acid in the stomach lining. These medications can be very effective and have a significant role in treating stomach ulcers in the acute setting. This led to their popularity among many providers as a go-to medicine for managing “gastric acid reflux.” Here, we will discuss the association between proton pump inhibitors and kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
Proton pump inhibitors (PPIs) use is so common. I am confronted with patients on long-term PPIs every day in my practice. The indication ranges from acid reflux to a history of gastrointestinal bleeding. These medications are now available over the counter and relatively inexpensive.
However, their widespread use of PPIs has aggravated many other medical issues. They are now linked to cardiovascular disease, osteoporosis, poor digestion, nutritional deficiencies, and kidney disease. While I am not a gastroenterologist, I will discuss the problems with their use and specifically discuss their effects on the kidneys.
The myth of acid reflux
The disease known as gastroesophageal reflux (GERD) is widespread, affecting more than 20% of the U.S. population. This disease which is the product of our modern living can lead to severe consequences, including esophageal narrowing and even cancer.
The main problem with GERD is that the sphincter that separates the stomach from the esophagus is leaky. So, the stomach's content in this disease is not going in the right direction. Instead, it is leaking to the esophagus.
GERD is, therefore, a motility problem, not an acid problem. However, current medical therapies focus on the acid part of the problem. Perhaps, this is because patients often feel a caustic burning sensation. This led to the proliferation of medications such as PPIs to suppress stomach acid production.
The role of stomach acid
Stomach acid plays an essential role in the digestion and absorption of food. This hydrochloric acid is produced by the cells that line the stomach. In conjunction with other enzymes, it acts as the first step in the digestive process. The acidity of the stomach also kills harmful bacteria.
In addition, stomach acid is vital for the digestion and absorption of protein, vitamin B12, iron, calcium and magnesium, and other minerals. Patients who lack stomach acid end up with various types of anemia and osteoporosis. Besides, poorly digested food can cause multiple symptoms and ferment, leading to the overgrowth of “bad” bacteria in the small intestine (SIBO).
The problem with the long-term use of a PPI
Long-term use of PPIs was associated with decreased bacterial richness and significant change in the gut microbiome. This has been linked to a decrease in the growth of “good” bacteria and an increase in “bad” bacteria. This dysbiosis is associated with many GI disorders, inflammation, and autoimmune diseases.
The use of PPIs was also linked to poor cardiovascular outcomes. They have been found to increase the risk of heart failure and death in patients with coronary artery disease. In a collective analysis of 23 studies (meta-analysis), PPIs also led to a 30% increase in major adverse cardiovascular events.
Furthermore, the long-term use of PPIs was connected to lower bone mineral density and a higher rate of osteoporosis. This is widely thought to be due to their effect on decreasing intestinal calcium absorption.
PPI use has also been linked to vitamin B12 deficiency, iron deficiency, and magnesium deficiency, among other nutritional deficiencies.
Proton pump inhibitors and kidney disease
PPI use has been linked to the development of acute interstitial nephritis (AIN, a kidney disease that affects the tubules and the tissue surrounding them). In fact, the use of PPIs was associated with a three-fold increase in the risk of AIN. This presentation is usually due to an immune response to PPIs and may improve with discontinuation. However, half of the patients may not recover and go on to develop CKD.
On top of that, the effects of PPIs on magnesium absorption cause magnesium deficiency. Low magnesium has been linked to various disturbances that can lead to CKD directly or indirectly.
PPI use was also associated with an increased risk for CKD, faster progression of CKD, and increased mortality in CKD patients.
Yet, all the studies I shared (via links) were observational. To date, there is no randomized controlled trial to confirm these findings. Such a study is likely unethical. Therefore, conventional researchers and authors suggest caution when reviewing this literature.
GERD, a motility disorder
Approaching the management of GERD as a motility disorder can significantly improve and even cure this disorder. This can be done by avoiding triggers such as foods, stomach over-distention, smoking, stress, and losing weight. Replacing nutritional deficiencies, eating more fiber, and digestive enzymes can also improve gastric emptying. The use of supplements, if necessary, can help symptom relief and repair the esophageal lining.
The bottom line
The long-term use of PPIs can be associated with various adverse outcomes. Observational studies demonstrated a link between using proton pump inhibitors and kidney disease. Patients taking PPIs for a long time without a specific indication should be reassessed. Gradual de-prescribing should be considered for these patients under an experienced Integrative medicine provider.
The Amazing Proximal Tubules
Each kidney contains several tiny filtering units called the nephrons. The nephron has two major sections: a filter, called the glomerulus (pleural: glomeruli), and the tubules. The tubules are divided into four sections. In this blog, we will focus mainly on the first section of the tubules, called the proximal tubules. We hope we will convince you that they are truly unique.
By Majd Isreb, MD, FACP, FASN, IFMCP
The proximal tubules
The proximal tubules are the site of retrieval of many electrolytes
The kidneys filter waste products from the blood in two stages. The first involves filtering a large amount of fluid through tiny blood vessels called glomeruli. In this first stage, 180-200 liters of blood are filtered daily! Considering that the total blood volume of the average adult is 4.5-5.7 liters, it is easy to realize that a person may die within a few hours if this was the only filtration step. Therefore, the second stage is so important. In it, millions of tiny tubes in the kidney, called tubules, reclaim most of that filtrate.
About 90% of this retrieval process occurs in the proximal tubule. The proximal tubules absorb 70% of the filtered sodium, 90% of the filtered bicarbonate, 60-70% of the filtered calcium, 70% of the filtered phosphate, and most of the filtered chloride. The proximal tubules also reclaim most of the filtered water (60%). In addition, up to 180 g/day of glucose is filtered by the glomerulus, and almost all of it is retrieved in the proximal tubule. The proximal tubules reabsorb all the filtered amino acids.
The proximal tubules are the site of many waste products and medication excretion.
Many small molecules are actively excreted in the proximal tubules. Drugs such as antibiotics, diuretics, nonsteroidal anti-inflammatory drugs, and others are excreted by the proximal tubules. Some of the gut-derived uremic toxins also get eliminated here. The proximal tubules are also the primary site for wasting metabolites such as folate, urate, creatinine, and carnitine. You can find a good list of these substances in this Wikipedia article.
Many specialized transporters are involved in this active transport. These include the families of organic anion transporters (OAT) and organic cationic transporters (OCT). Other transporters are also involved. It is, therefore, easy to understand that minor genetic changes in the genes that encode these transporters can lead to significant kidney diseases, including kidney stones.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The site of gluconeogenesis in the kidneys
The levels of blood sugar (glucose) levels are heavily regulated. Gluconeogenesis describes the process our body produces glucose from other precursors. This process is crucial for maintaining blood sugar balance in normal individuals. The liver is usually known to be the primary site for gluconeogenesis. However, recent data showed that the kidneys are also a significant site for gluconeogenesis.
After an overnight fast, the kidneys account for 40% of gluconeogenesis. Most of that is produced in the proximal tubules. The proximal tubules use lactate to produce glucose. This process is regulated by insulin, blood acid level, and stress hormones.
Cortisol appears to increase the production of glucose by the kidneys. Epinephrine (the fight or flight hormone) also can increase glucose release by the kidneys. This effect was eliminated by renal denervation. Insulin, on the other hand, decreases the release of glucose by the kidneys. In addition, elevated blood and urine glucose was found to reduce glucose production in the proximal tubules. Finally, high blood acid level has been found to increase glucose production by the kidneys.
The place of insulin degradation
Since we are talking about glucose balance, we should add that the kidneys are a significant site for insulin metabolism and clearance. The kidneys are responsible for 40% of insulin metabolism and clearance. In healthy individuals, the kidney clearance of insulin is 200 ml/min. This value is greater than GFR. This is because the glomeruli filter insulin. The proximal tubules then reabsorb it. Most of that gets broken down in the proximal tubular cells.
The proximal tubules are responsible for kidney clearance. This explains the insulin requirement decrease in diabetic patients who develop advanced kidney disease. It is also why many diabetic patients with progressive kidney disease develop low blood sugar while on glucose-lowering medications.
The site of vitamin D activation in the kidneys
Vitamin D is a fat-soluble vitamin essential for life and crucial for calcium balance and bone health. It is produced by our skin or consumed in the diet and then transported to the liver as a “prohormone” by a protein called Vitamin D Binding Protein (DBP). In the liver, this precursory form is converted to 25-hydroxyvitamin D (abbreviated 25(OH)D). 25-(OH)D is the primary circulating form of vitamin D. 25(OH)D is eventually transported to the kidneys, where another (-OH) group is added. The result is the formation of the active hormone called 1,25-dihydroxy vitamin D (or 1,25(OH)2D).
The kidneys not only convert 25(OH)D to the active hormone 1,25 (OH)2D, but they also reclaim 25(OH)D that is filtered in the first stage. The majority of this happens in the proximal tubules. In CKD, this process declines. This explains the high rate of vitamin D deficiency in CKD. In healthy individuals, when the proximal tubules reabsorb 25(OH)D, it gets either converted to 1,25(OH)2D or recycled back into the circulation.
Tubular secretion of creatinine
As we mentioned above, the proximal tubules secrete creatinine by OCT transporters. This secretion was found to increase with the worsening kidney function. Kidney function in clinical practice is measured by estimating the glomerular filtration rate (eGFR) based on serum creatinine levels. Since these levels are inaccurate due to increased tubular secretion of creatinine, studies have looked at another blood marker that is only filtered by glomeruli and not affected by the tubules. Cystatin C is one of these markers.
Testing tubular secretory function
Despite the importance of tubular secretory function, it is rarely measured or even estimated. The development of accurate and valuable tests of tubular secretion faces several challenges. The major challenge is finding a substance exclusively eliminated by the proximal tubules.
However, testing and measuring the proximal tubules' secretory function is very important. It can:
- Predict the clearance of gut-derived uremic toxins.
- Allow early identification of specific kidney diseases
- Optimize medication dosing
- Estimate residual kidney function
- Predict metabolic complications of kidney disease
The bottom line
The proximal tubules are often underestimated and misunderstood in kidney health and disease. These sections of the kidneys are highly active and perform many biological functions. This explains why they utilize high energy and require significant nutritional and antioxidant support.
August Research and News
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney August Research and News.
Uric acid-lowering medications don't prevent CKD
Uric acid is a waste metabolite produced from the breakdown of purines. Elevated serum uric acid levels are associated with a higher risk of hypertension, cardiovascular disease, and mortality and progression of chronic kidney disease (CKD).
This study was an observational retrospective evaluation aimed to examine the association between initiating uric acid–lowering therapy and the incidence of CKD. The study was published in June in JAMA Network Open.
269,651 patients with an estimated glomerular filtration rate of ≥60 mL/min/1.73 m2 and no albuminuria were assessed. These patients were treated at the US Department of Veterans Affairs health care facilities from 2004 to 2019.
There was no beneficial association between initiating uric acid–lowering therapy and the incidence of CKD. In fact, uric acid lowering–therapy was associated with a significantly higher risk of new-onset CKD.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Discontinuing fenofibrate decreases serum creatinine
This prospective observational small study evaluated patients who were referred to a nephrology clinic for a new evaluation of a rise in serum creatinine or worsening chronic kidney disease who were on fenofibrate.
Fenofibrate is an anti-lipidemic agent prescribed to lower high cholesterol and triglyceride levels.
Discontinuation of fenofibrate resulted in a sustained reduction in serum creatinine in about half of the patients and for up to 1 year. Median serum creatinine at the time of fenofibrate discontinuation was 1.9 mg/dL.
Upon discontinuation of fenofibrate, the median serum creatinine decreased to 1.5, 1.4, and 1.4 mg/dL at 3, 6, and 12 months respectively.
This study confirms previous reports that fenofibrate increases serum creatinine without causing kidney disease. This was thought to be due to an increase in the metabolic production of creatinine.
Music for kidney health
This featured article published in CJASN examined the health implications of music. The authors discussed the impact of music on the mind and body and its ability to help ease pain and fear.
According to the authors, in addition to conventional pharmacological treatment, music can play a complementary role as an affordable non-pharmacologic therapeutic tool to improve quality of life and potentially reduce morbidity and mortality in chronic kidney disease patients.
The authors cited several studies and discussed how music can potentially help with managing depression, anxiety, and pain, and improving the quality of life for kidney patients.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Homemade Almond Milk
In this new category, we will post once a month practical tips and tricks for healthy living with kidney disease. Consider these as tools that you can modify according to your unique situation. These tools span across our principles of Integrative medicine approach for kidney disease, including genetics, environmental exposure, gut health, lifestyle, and others. We invite others to contribute to this category. If you are interested in contributing, please contact us info@inkidney.com. In this first blog, we will share a recipe for making homemade almond milk.
Plant-dominant diets have been shown to delay the progression of kidney disease. With proper implementation, eating plant-dominant provides a good source of alkali-promoting foods without significant risk of raising potassium or phosphorus levels.
Ideally, a plant-based diet should include 8-10 servings per day primarily of vegetables, with some fruits. For any given meal, vegetables and fruits should be the main dish with animal protein, if included, as a side dish, not the main attraction. This is often the opposite of how many people typically center on meat or poultry as the main event, with vegetables serving as sides. Fruit is also recommended, choosing those lower in carbohydrates, and higher in fiber and phytonutrient benefits.
Homemade Almond Milk
Evidence indicates that dairy milk is a high source of phosphate. Elevated phosphate burden is associated with poor outcomes for patients with Kidney disease. We prefer homemade almond milk over store almond milk which may contain preservatives.
Join us in the fight against kidney disease and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Almond milk has high oxalate content (68 mg/100 mL). So, if you have oxalate-containing kidney stones or elevated urinary oxalate, we recommend avoiding almond milk. Ideal use for it is as a part of a plant-based shake that contains other ingredients that bind oxalate in the gut.
Homemade Almond Milk
Directions
- Soak 1 cup of almonds in filtered water overnight.
- Drain the almonds and add them to the blender with 3 Medjool dates, 1 whole chopped vanilla bean, and 3 ½ cups of filtered water.
- Blend on high for 1-2 minutes until the milk looks nice and creamy.
- Place a mesh strainer over a glass jug and slowly pour the milk through.
- Store in the fridge for 3-5 days and shake well before each use.
Enjoy!
The Role of Kidneys in Sugar Balance
The levels of blood sugar (glucose) are heavily regulated. When we eat carbohydrates (carbs), we break them down in the gut into simple sugars. These sugars all turn into glucose which circulates in the bloodstream. When glucose is absorbed into the bloodstream, the pancreas produces insulin. Glucose is used by various cells and organs as a major source of energy. In this blog, we will discuss the role of kidneys in sugar balance.
By Majd Isreb, MD, FACP, FASN, IFMCP
Glucose metabolism
Glucose metabolism is a very complex process. Books have been written about it. So, it will not be fair to cover it and understand it in a simple paragraph in this blog. However, it is useful to divide glucose metabolism into multiple processes:
- Glycolysis: This is the process of breaking down glucose to produce energy and other metabolic products. It usually occurs in the liver after eating carbs.
- Gluconeogenesis: In this process, the liver makes glucose from other substances such as fats and proteins after periods of fasting.
- Glycogenesis: Here, excess glucose is stored in the liver and muscle cells as glycogen.
- Glycogenolysis: During fasting, the liver and muscles can release stored glycogen into the bloodstream to maintain stable blood glucose levels.
Normally, glucose blood levels are tightly regulated. Insulin is produced by the pancreas after glucose is absorbed into the bloodstream. Insulin facilitates the entry of glucose into the cells. It also prompts the liver to store glucose in the form of glycogen. This is why it is crucial for regulating blood glucose levels. Insulin has many other roles such as protein building in the muscles.
During periods of fasting such as overnight sleep, the pancreas produces another hormone called glucagon which triggers the liver and muscles to release stored glycogen to maintain blood glucose levels. Other hormones involved in glucose regulation include cortisol, amylin, incretins, ghrelin, leptin, adiponectin, and growth hormone.
Traditionally, it has been thought that the liver and pancreas are the major organs involved in glucose regulation. The gut, fat, and muscle cells also play a role. However, little is known about the role of the kidneys in sugar balance.
The role of kidneys in sugar balance
Gluconeogenesis in the kidneys
Gluconeogenesis describes the process our body produces glucose from other precursors. This process is crucial for maintaining blood sugar balance in normal individuals. The liver is usually known to be the primary site for gluconeogenesis. However, recent data showed that the kidneys are also a significant site for gluconeogenesis.
After an overnight fast, in fact, the kidneys account for 40% of gluconeogenesis. Most of that is produced in the proximal tubules. The proximal tubules use lactate and amino acids to produce glucose. This process is regulated by insulin, blood acid level, and stress hormones.
Cortisol appears to increase the production of glucose by the kidneys. Epinephrine (the fight or flight hormone) also can increase glucose release by the kidneys. This effect was eliminated by renal denervation. Therefore, it appears that kidneys play an important role in maintaining glucose levels in critical illnesses or under stress.
Insulin, on the other hand, decreases the release of glucose by the kidneys. In addition, elevated blood and urine glucose was found to reduce glucose production in the proximal tubules. Finally, high blood acid level has been found to increase glucose production by the kidneys.
Interestingly, SGLT2 inhibitors which have recently gained popularity as medications that improve cardiac and renal outcomes increase gluconeogenesis in the kidneys.
The place for insulin degradation
Since we are talking about glucose balance, we should add that the kidneys are a significant site for insulin metabolism and clearance. The kidneys are responsible for 40% of insulin metabolism and clearance. In healthy individuals, the kidney clearance of insulin is 200 ml/min. This value is obviously greater than GFR. This is because the glomeruli filter insulin. The proximal tubules then reabsorb it. In addition, insulin enters the tubular cells directly from their surrounding blood vessels. Most of the insulin that enters proximal tubular cells directly or by reabsorption gets broken down and degraded.
The proximal tubules are responsible for 60% of insulin metabolism and clearance by the kidneys. This explains the decrease in insulin requirement in diabetic patients who develop advanced kidney disease. The loss of kidney function decreases the degradation of insulin. Therefore, more insulin will be available in the circulation. This is also why many diabetic patients with progressive kidney disease develop low blood sugar while on glucose-lowering medications.
The bottom line
The kidneys play an important role in maintaining blood glucose levels, especially during stress and critical illness. Patients with advanced kidney disease require less insulin to control their blood sugar levels because of the loss of the normal abilities of the kidneys to break down insulin.
Cadmium and kidney disease
According to the CDC, 1 in every seven people in the US has CKD. The majority of risk factors for CKD are related to lifestyle factors. Exposure to environmental chemicals and toxins is associated with the development and progression of kidney disease. Here, we will focus on cadmium and kidney disease.
Cadmium is naturally occurring in the environment as a pollutant derived from agricultural and industrial sources. Long-term exposure to cadmium leads to many adverse outcomes such as cancer and organ toxicity.
Cadmium and kidney disease
By Majd Isreb, MD, FACP, FASN, IFMCP
Source of Exposure
Exposure to cadmium mainly occurs through ingesting contaminated food, water, or inhalation. The most significant sources of cadmium exposure include:
- Batteries
- Pigments and coatings
- Plastics
- Phosphate fertilizers
- Tobacco cigarettes
Although there are more toxic chemicals, we are exposed to more cadmium. Phosphate fertilizers, for example, can lead to soil and water contamination with subsequent accumulation in plants, mainly root vegetables (carrots, etc.) Cadmium accumulates in these plants with a half-life of 25-30 years. In addition, it is estimated that individuals who smoke one pack of 20 cigarettes each day will absorb approximately 1–2 µg of Cadmium daily.
How does the body handle cadmium?
Cadmium absorption varies depending on the route of exposure. For example, absorption after inhalation is higher than that from food ingestion. Cadmium concentrations in the blood can be up to four or five times higher in tobacco smokers.
Normal individuals only absorb 6% of ingested cadmium. However, the absorption increases in those with iron deficiency. This is because cadmium uses the same transporter as iron (divalent metal transporter 1, DMT1) which is upregulated with iron deficiency. This also explains why women have higher levels of cadmium than men.
Cadmium has no biological function in the body. Once absorbed, it binds to metallothionein in the liver. Metallothioneinsare cysteine-rich, small metal-binding proteins that are present in the cell. They help balance metals such as zinc and copper in the body. They also protect against the toxicity of cadmium and other heavy metals.
The biggest problem with cadmium is its long half-life of 20-40 years. This is likely due to its incorporation into the bone.
Join us to end the kidney disease epidemic
How the kidneys handle cadmium
The human body has no effective way to eliminate cadmium. When cadmium-metallothionein compound reaches the kidneys, it is filtered freely by the glomeruli. The problem is that most of that compound gets reabsorbed by the proximal tubular cells of the nephron. Inside these cells, cadmium is dissociated from metallothionein and reenters the circulation. There are other ways for cadmium to enter the tubular cells. This includes active transport into the tubular cells via the organic cation transporter 2. This makes the proximal tubules the primary site of cadmium-induced kidney injury. The kidneys accumulate 50% of the total body burden of cadmium in chronic exposure.
Testing for exposure
Blood cadmium levels are usually checked in cases of acute ingestion and toxicity. However, we are primarily concerned with chronic low-level exposure to cadmium. For that purpose, measuring urinary cadmium reflect the total body burden and long-term exposure. The level is checked on a single spot urine sample and is reported as a ratio to urinary creatinine. The result is usually expressed as mcg/g. Ideally, there should be no normal urinary cadmium, but according to the CDC,a level of < 0.19 mcg/g s normal. The level increases with age. In patients with CKD, urinary levels may not be reliable and may not reflect long-term exposure.
The effects of cadmium on the kidneys
Indirect
Several studies have shown that chronic exposure to cadmium increases the risk of developing diabetes and the severity of established diabetes. Cadmium appears to increase insulin release, increase insulin resistance, and enhance gluconeogenesis. Cadmium also increases the risk of developing diabetic kidney injury.
Cadmium exposure was associated with higher blood pressure and increased prevalence of hypertension in an analysis of the NHANES study. This is thought to be due to increased renin activity and increased reactivity to vasomotor stimulation.
Finally, cadmium was found to increase the risk for obesity. The presence of cadmium in maternal blood during pregnancy was also associated with the offspring.
Direct
As we saw above, the kidneys are one of the major organs affected by cadmium. The proximal renal tubules are the primary site of that injury. This leads to a decrease in the absorption of some proteins, glucose, amino acids, phosphate, and calcium. This syndrome is called Fanconi syndrome. Beta 2-microglobulin (B2M) is a protein typically reabsorbed in the tubules. The presence of B2M in the urine can be a marker for early cadmium kidney injury. Ultimately, the glomeruli are injured, and kidney function declines.
Even low-level exposure to cadmium increases the risk for chronic kidney disease.
The toxic effects of cadmium on the kidneys can be summarized as follows:
- Decreased kidney function
- Tubular injury (Fanconi’s Syndrome: loss of glucose, amino acids, phosphate, and calcium in the urine)
- Presence of protein in the urine
- Increase risk for kidney stones (due to increased calcium in the urine)
- Decreased activation of vitamin D, which usually occurs in the tubules
- Oxidative stress
- Faster progression of CKD from other causes
Nutrients and cadmium exposure
As mentioned above, iron deficiency increases the risk of cadmium exposure and toxicity. Adequate iron supplementationhas been found to decrease cadmium retention.
In addition, zinc has been found to protect the kidneys from cadmium injury. Zinc competes with cadmium for entry into cells. It has also been found to decrease cadmium-induced apoptosis in kidney cells.
Magnesium was also found to decrease cadmium toxicity. This is thought to be due to competition with cadmium decreasing its uptake by the cells. It stimulates glutathione synthesis and reduces oxidative stress.
Finally, animal studies showed a protective role of selenium against cadmium toxicity in the liver and kidney. The positive effect of selenium is mainly due to the increased activity of antioxidant selenoproteins such as glutathione peroxidase.
Genetics and cadmium exposure
There is not much literature about the role of genetic variation and the risk of cadmium exposure. However, it is conceivable that genetic variants in the metallothionein 2 A gene can be associated with a higher risk of cadmium toxicity. Other candidate genes affecting the risk for cadmium toxicity include the DMT1 gene, glutathione-related genes, and methylenetetrahydrofolate reductase.
The microbiome and cadmium exposure
Cadmium impacts the gut in two ways. On one side, exposure to cadmium causes significant changes to the gut microbiome (increased Bacteroidetes-to-Firmicutes ratio). It also leads to an increase in lipopolysaccharide (LPS) production. On the other side, it produces an inflammatory response, cell damage, and disruption of tight junctions. Cadmium, therefore, leads to increased intestinal permeability (leaky gut). This will lead to systemic inflammation and increase the risk for autoimmune diseases.
Protecting the body from cadmium
Optimizing nutritional status is essential for decreasing cadmium absorption and its effects. Several steps can be taken to reduce exposure to cadmium with its negative impact on kidney health. These include:
- Stop smoking!
- Choose a home filtration device that effectively removes heavy metals, including cadmium. Not all consumer filters will remove cadmium, consider upgrading to Clearly filtered, Multipure, and Aquasauna water filter systems.
- Always choose organic produce whenever possible. Conventionally grown produce fertilized with phosphate fertilizers may contain high levels of cadmium.
- Antioxidants are protective against Cadmium damage, so a diet high in antioxidants like citrus fruit, green tea, blueberries, and dark chocolate will be naturally protective. Enhance detoxification by eating dark leafy greens, cruciferous veggies (like broccoli, kale, and cabbage), and sulforaphane-rich foods like garlic and onion contain high concentrations of nutrients that increase our detoxification capacity.
- Eat a well-balanced plant-dominant diet that contains calcium, iron, magnesium, zinc, and selenium.
- Several lifestyle modifications can support improved detoxification, including Epsom-salt baths, sauna, lymphatic massage, sweat-inducing workouts, and dry brushing.
The bottom line
Most cadmium exposure is due to smoking cigarettes and food ingestion. Cadmium exposure is a huge problem due to its long half-life and kidney concentration. It causes significant kidney damage, especially in the tubules. Nutritional deficiencies can enhance its toxicity, but you can take steps to reduce your risks.
July Research and News
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney July Research and News.
Diet therapy along with nutrition education can improve renal function in people with stage 3-4 CKD who do not have diabetes
In this randomized controlled trial published in the British Journal of Nutrition, investigators assessed the role of individualized dietary therapy and counseling on eGFR.
One hundred twenty CKD patients who don't have diabetes were randomized into an intense nutrition group and a controlled group (routine and standard care) for six months. The intense nutrition group had a significantly lower protein and sodium intake than the control group.
This was associated with a statistically significant improvement in the intense nutrition group's eGFR, creatinine, and blood pressure control. Depression score was also improved.
The study again highlights the importance of dietary intervention in CKD and the need for coaching and counseling patients for at least six months to make an impact.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Consuming nuts 1-6 times a week is associated decreased risk of CKD
Researchers in this study found that consuming nuts 1-6 times a week was associated with lower risks of chronic kidney disease (CKD) and all-cause mortality.
The study examined 6,072 adults who participated in the NHANES 2003–2006. Consuming nuts 1-6 times a week was associated with a significant reduction in the prevalence of CKD.
Both patients who did or did not develop CKD had a significant decrease in all-cause mortality.
This study emphasizes the importance of a plant-dominant diet in decreasing the incidence of CKD regardless of the oxalate content in the plant sources. It is well known that plant-based and plant-dominant diets also delay the progression of CKD.
Genetics + environment = lupus nephritis
A recent article in Clinical Kidney Journal summarizes recent reports describing genetics' role in causing lupus nephritis.
The authors describe how loss-of-function variants of Tumor Necrosis Factor Alpha-Induced Protein 3 (TNFAIP3) and gain of function variants of Toll-like receptor 7 (TLR7) cause lupus and lupus nephritis. Both lead to NF-ĸB activation.
This follows another recent study published in Nature that demonstrated that a gain of function genetic variant in TLR7 causes human lupus.
TLR7 is a sensor of viral RNA.
This highlights the interaction between genetics and environmental triggers in lupus nephritis and other autoimmune kidney disorders.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.