Is Organic Food Better for Kidney Health?
In recent years, there has been growing interest in understanding the relationship between diet and kidney health, particularly the role of organic food. This blog explores the question: Is organic food better for kidney health? We delve into the definitions, nutritional aspects, and research findings to provide a comprehensive view.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is organic food? Understanding standards and certifications
Organic food, as defined by the U.S. Department of Agriculture (USDA), adheres to stringent farming and processing standards focused on natural methods and environmental sustainability. For crops, this includes using land free from synthetic fertilizers and pesticides for at least three years, employing natural soil fertilization and pest control methods, and avoiding genetic modification.
In the case of livestock, organic standards require outdoor access for animals and organic feed and restrict the use of antibiotics and growth hormones. The processing of organic foods prohibits artificial preservatives, colors, or flavors and avoids irradiation and industrial solvents.
The certification process for organic food involves rigorous checks by USDA-accredited agents, ensuring adherence to organic regulations and annual inspections. Products entirely composed of organic ingredients can be labeled '100% organic,' while multi-ingredient products require at least 95% organic content to use the USDA organic seal. These standards aim to promote ecological balance, conserve biodiversity, and contribute to the overall quality and safety of food consumption.
Join us to end the kidney disease epidemic
Impact of pesticides in conventional foods on kidney health
Pesticides and herbicides used in conventional farming are a growing concern for public health, particularly regarding their potential impact on kidney health. These chemicals, designed to protect crops from pests and diseases, can leave residues in food products.
When consumed, these residues can accumulate in the body over time. Research has shown that certain pesticides can have nephrotoxic effects, meaning they can damage or impair the function of the kidneys. This is particularly concerning because the kidneys' primary function is to filter and eliminate toxins from the body. Prolonged exposure to certain pesticides has been associated with chronic kidney disease, especially in agricultural communities where direct exposure is more common.
Analysis of the National Health and Nutrition Examination Survey (NHANES) data in the United States showed that people who are exposed to pesticide malathion are at a higher risk of developing serious kidney disease.
The benefits of choosing organic foods, which have lower pesticide residues, become particularly relevant in this context. Organic farming standards prohibit or strictly limit the use of synthetic pesticides, leading to significantly lower levels of these chemicals in organic produce compared to conventionally grown foods.
For individuals with existing kidney issues or those at risk, reducing exposure to these potential toxins by consuming organic foods could be beneficial. These individuals may suffer from increased concentrations of pesticides related to accumulation from decreased clearance by the kidneys.
While more research is needed to fully understand the long-term impacts of pesticide residues on kidney health, the current evidence suggests that minimizing exposure through organic food choices is a prudent step for those concerned about their kidney health.
Nutritional benefits of organic food in kidney health
Organic foods are often touted for their higher nutritional content, which can have significant implications for kidney health. Studies have shown that organic produce can contain higher levels of certain nutrients, antioxidants, and omega-3 fatty acids.
Antioxidants, for instance, play a crucial role in reducing oxidative stress in the body, a condition that can exacerbate kidney damage, particularly in chronic kidney disease (CKD) patients. Oxidative stress is a known contributor to the progression of kidney diseases and related complications. Therefore, the higher antioxidant levels found in organic fruits and vegetables might help in mitigating these risks.
Another vital nutrient found in higher quantities in organic food is omega-3 fatty acids, particularly in organic meats and dairy products. Omega-3s are beneficial for heart health, and emerging research suggests they can also play a role in kidney health. They are known to have anti-inflammatory properties, which can be beneficial in reducing the risk of kidney diseases and slowing their progression.
For people with kidney issues, inflammation is a key concern, and managing it through dietary choices can be a practical approach. Additionally, organic foods are less likely to contain harmful pesticides and chemicals that could accumulate in the body and potentially harm the kidneys.
Heavy metals in conventional vs. organic foods
Organic foods might have a more favorable balance of certain minerals important for kidney health. For example, they may have lower levels of harmful heavy metals like cadmium, which is detrimental to kidney health. Cadmium can accumulate in the kidneys and potentially lead to kidney damage over time. Organic farming practices, which prohibit the use of synthetic fertilizers, might contribute to lower cadmium levels in organic produce.
Dietary Recommendations: Balancing Organic Food and Kidney Health
The decision to choose organic foods also depends on other factors, including cost and availability. Organic foods often come with a higher price tag, which can be a barrier for many people. A practical approach for those unable to switch entirely to organic is to focus on avoiding the "dirty dozen" - a list of fruits and vegetables known to have higher pesticide residues.
The bottom line
While organic foods offer certain nutritional benefits and lower pesticide and heavy metal exposure, the direct impact on kidney health is not clearly defined in the conventional peer-reviewed literature. It's important for individuals, especially those with kidney issues, to consider all aspects, including nutritional content, potential contaminants, cost, and personal health goals, when making dietary choices.
January Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the January Research and News.
Happy New Year!
Varied Treatment Effects of Intensive Glycemic Control in ACCORD
A post-hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial by Charu et al. examines the variable effects of intensive glycemic control on kidney microvascular outcomes and mortality in patients with type II diabetes.
The study utilized the kidney failure risk equation (KFRE) to stratify the ACCORD trial population into quartiles based on their 5-year kidney failure risk.
The analysis revealed that the benefits and risks of intensive glycemic control varied with the baseline risk of kidney failure. Patients with a higher baseline risk derived more benefit in terms of reducing kidney microvascular outcomes (with a 7-year restricted-mean-survival-time [RMST] difference of 114.8 days) compared to the overall trial population (48.4 days).
However, this group also experienced a significantly shorter time to death (7-year RMST difference of -56.7 days) compared to the overall effect in the trial (-23.6 days).
Why is this important
This study highlights the importance of individualizing glycemic targets in patients with type II diabetes, especially those with varying risks of kidney failure.
The findings suggest that while intensive glycemic control can be more beneficial for patients at higher risk of kidney microvascular complications, it may also increase their risk of mortality.
This underscores the need for careful patient selection and risk stratification when considering intensive glycemic control strategies.
Understanding the heterogeneous effects of such treatments can guide clinicians in balancing the benefits and risks, ultimately leading to more personalized and effective diabetes management.
Join us to end the kidney disease epidemic
Vitamin K2 Supplementation in Dialysis Patients Did Not Lead to Increased Coagulation
This study is coming from the dialysis world. It was published ahead of print in the Journal of Renal Nutrition.
The study focused on the effects of vitamin K (MK-7) supplementation on patients undergoing dialysis, who typically exhibit poor functional vitamin K status, and aimed to assess the potential procoagulant effects of such supplementation.
The double-blinded, placebo-controlled trial involved 123 dialysis patients randomized to receive either vitamin K (MK-7, 360 ug/daily, n=61) or a placebo (n=62) for a period of 52 weeks.
Various biomarkers of coagulation, including thrombin generation and clot activities of vitamin K-dependent coagulation factors, were measured at the beginning and end of the intervention. Additionally, vascular adverse events (AE) and serious adverse events (SAE) were monitored.
The results showed a significant decrease in PIVKA-II, a marker of vitamin K deficiency, in the vitamin K group compared to the placebo group after one year.
However, no significant between-group differences or within-group changes were observed for other biomarkers of coagulation. Importantly, there were no differences in AE and SAE, including vascular events or death, between the two groups.
Why is this important
This study's findings are significant as they indicate that a one-year regimen of vitamin K2 supplementation does not lead to detectable effects on coagulation activation or increase the risk of vascular events or death in patients on dialysis.
This addresses a crucial concern about the potential procoagulant effects of vitamin K2 supplementation in this vulnerable patient group. By demonstrating the safety of vitamin K2 supplementation, this research could pave the way for its wider use in managing functional vitamin K deficiency in dialysis patients, potentially helping to mitigate the risk of vascular calcification associated with this condition.
We believe that supplementing vitamin K2 should go hand in hand with adequate supplementation of vitamin D3 and starts earlier in chronic kidney disease (as early as stage 2).
D-Alanine's Impacts the Circadian Clock and Glucose Metabolism in the Kidneys
Sakai et al.'s study investigates the role of d-alanine in regulating glucose metabolism through the circadian clock in kidneys.
Using high-performance liquid chromatography, the study measured d-alanine in mice blood and urine, discovering a clear intrinsic circadian rhythm. This rhythm was regulated by the kidney through urinary excretion.
In the kidneys, d-alanine-induced genes that are involved in gluconeogenesis and circadian rhythm, notably stimulating glucose production in mice, especially in kidney proximal tubular cells, but not in liver cells.
The study also explored the intraday variation in the gluconeogenetic effect of d-alanine, partially mediated through the circadian transcriptional network. Under constant darkness, d-alanine normalized the circadian cycle of behavior and kidney gene expressions.
Why this is important
This research is significant as it sheds light on a novel aspect of kidney physiology and its impact on systemic glucose metabolism.
The findings that d-alanine influences gluconeogenesis and circadian rhythm in the kidney provide new insights into how circadian rhythms regulate metabolic processes, especially in conditions like diabetes where glucose regulation is disrupted.
Understanding the role of d-alanine could lead to innovative therapeutic strategies for managing glucose metabolism in lifestyle-related diseases and benefiting shift workers, whose natural circadian rhythms are often disrupted.
This study also opens up new avenues for exploring dietary and pharmacological interventions targeting circadian rhythms to improve metabolic health.
The Clinical and Pathological Characteristics of Patients with Oxalate Nephropathy
In a study conducted at the Cleveland Clinic from January 2015 to March 2023, Llanos et al. examined the clinical and pathological characteristics of oxalate nephropathy (ON), a condition marked by the deposition of calcium oxalate crystals in the kidneys.
The study, one of the largest of its kind, analyzed 60 native kidney biopsies, ultimately focusing on 31 patients after excluding those with clinically insignificant biopsies or lacking data.
The mean age of patients at diagnosis was 66.2 years, with a slight female majority. Common comorbidities included hypertension (87.1%), diabetes (58.1%), nephrolithiasis (42%), and chronic kidney disease (77.4%).
The biopsies revealed abundant calcium oxalate crystal deposits, with acute tubular injury and diabetic glomerulosclerosis being the most frequent additional diagnoses. Notably, interstitial fibrosis was present in a significant number of cases.
The study observed that oxalate nephropathy often presented as acute kidney injury (AKI) or acute on chronic kidney disease (CKD), with a poor prognosis.
Most patients required dialysis at presentation, and the mortality rate was high. The study also noted an increase in vitamin C intake post-COVID pandemic, which might contribute to the condition.
Why this is important
This study is crucial as it provides a detailed understanding of oxalate nephropathy, a condition that is often underrecognized yet has significant implications.
The findings highlight the severity of ON, with high rates of dialysis dependency and mortality, stressing the need for early recognition and management.
Understanding the risk factors and clinical course of ON can aid clinicians in prompt diagnosis and tailored treatment strategies, potentially improving patient outcomes.
Additionally, the study's insight into the potential role of dietary factors, such as increased vitamin C intake, in the development of ON is particularly relevant in the context of changing dietary habits and the post-COVID-19 pandemic era.
Review article of the month
Exercise and Kidney Disease
While there is still a need for further research in this field, there is a tremendous body of evidence supporting the role of exercise in slowing the progression of kidney disease.
This review article summarizes all the studies about the benefits of exercise in kidney disease.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
B Vitamins: Energizing Allies in Kidney Health
The kidneys are vital organs requiring significant energy, accounting for about 7% of resting energy expenditure in healthy individuals. B vitamins, particularly nicotinamide (B3) and riboflavin (B2), are essential for mitochondrial energy production in the kidneys.
Understanding chronic kidney disease and nutritional needs
In Chronic Kidney Disease (CKD), there is an increased energy requirement due to heightened inflammation and oxidative stress, particularly at the level of the renal tubules. This increased metabolic demand amplifies the need for B vitamins, which are crucial cofactors in numerous energy-producing reactions and in bolstering antioxidant defenses, like the activity of glutathione.
The impaired kidney function in CKD leads to a greater necessity for these vitamins to cope with the additional stress and to support overall cellular health and function. Addressing these heightened nutritional needs is vital in managing CKD effectively, emphasizing the importance of a diet or supplementation plan that adequately supplies these essential nutrients.
Food sources of vitamin B complex
B vitamins are found in a wide range of foods, making them accessible through a well-balanced diet. Here are some common food sources for various B vitamins:
- Vitamin B1 (Thiamin): Whole grains, meat (especially pork), nuts, and seeds.
- Vitamin B2 (Riboflavin): Dairy products, eggs, lean meats, green vegetables, and fortified cereals.
- Vitamin B3 (Niacin): Poultry, beef, fish, whole wheat bread, peanuts, and fortified cereals.
- Vitamin B5 (Pantothenic Acid): Chicken, whole grains, broccoli, mushrooms, and avocados.
- Vitamin B6 (Pyridoxine): Fish, beef liver, potatoes, and non-citrus fruits.
- Vitamin B7 (Biotin): Eggs, almonds, spinach, sweet potatoes, and cheese.
- Vitamin B9 (Folate): Leafy green vegetables, legumes, seeds, and liver.
- Vitamin B12 (Cobalamin): Animal products like meat, fish, poultry, eggs, and dairy. (Note: Vitamin B12 is not naturally found in plant foods.)
Dietary restrictions in advanced kidney disease and B vitamin deficiencies
Individuals with advanced kidney disease often face dietary restrictions that can impact their intake of B vitamins. These restrictions might include limitations on protein, potassium, phosphorus, and fluids, which can inadvertently reduce the consumption of B vitamin-rich foods.
- Protein-Restricted Diets: Often recommended in CKD to reduce kidney workload, protein restriction can limit sources of several B vitamins, especially B12 found in animal products.
- Potassium and Phosphorus Limitations: Foods high in potassium and phosphorus, like certain fruits, vegetables, nuts, and dairy products, may also be sources of B vitamins. Restricting these can inadvertently decrease B vitamin intake.
These dietary limitations highlight the importance of carefully planning meals and possibly supplementing B vitamins under medical supervision. It's crucial for individuals with CKD to work closely with an Integrative dietitian to balance their nutritional needs while adhering to dietary restrictions. This approach ensures adequate intake of essential nutrients, including B vitamins, without overburdening the kidneys.
Recommended vitamin B complex for CKD patients
When considering vitamin B complex supplementation for Chronic Kidney Disease (CKD) patients, it's essential to recognize the unique nutritional needs due to the disease's impact on the body's ability to process nutrients. The waste buildup in CKD patients can change how the body uses vitamins and minerals, leading to deficiencies, particularly in B vitamins. Given the crucial role of B vitamins in cellular functions, energy production, and red blood cell formation, managing their levels is vital for CKD patients.
For CKD patients, a daily vitamin B complex supplement may be recommended. This complex typically includes vitamins B1 (thiamin), B2 (riboflavin), B6, B12, folic acid, niacin, pantothenic acid (B5), biotin, and a small dose of vitamin C. However, the specific dosages may vary based on individual health conditions, CKD stage, and other factors.
- Vitamin B1 (Thiamin): Helps cells produce energy from carbohydrates and supports the nervous system. A supplement of 1.5 mg/day is often recommended in addition to daily dietary intake.
- Vitamin B2 (Riboflavin): Assists in energy production, supports vision, and maintains healthy skin. A dose of 1.8 mg/day is usually suggested for CKD patients on a low-protein diet, and 1.1-1.3 mg/day for those on dialysis.
- Niacin (Vitamin B3): Vital for using sugars and fatty acids, and aids in cellular energy production. Recommended supplementation is 14 to 20 mg/day.
- Vitamin B6: Important for protein metabolism and red blood cell production. Suggested doses are 5 mg/day for non-dialysis CKD patients and 10 mg/day for those on dialysis.
- Folate (Vitamin B9): Essential for DNA synthesis and red blood cell formation. A typical recommendation is a 1.0 mg/day supplement.
- Vitamin B12: Crucial for new cell formation and nerve health. The suggested supplement is 2-3 ug/day.
- Biotin and Pantothenic Acid: These vitamins aid in energy production and metabolism of proteins, fats, and carbohydrates. A daily supplement of 30-100 ug for biotin and 5 mg for pantothenic acid is often recommended.
It's important to note that CKD patients need to be cautious with fat-soluble vitamins (especially vitamins A and E) as they can accumulate in the body. These should only be taken under medical supervision and with follow-up on their levels.
Given the complexity of CKD and the potential for nutrient imbalances, it is critical for patients to work closely with their Integrative healthcare providers, including nephrologists and renal dietitians, to determine the most appropriate supplementation plan. This tailored approach ensures that the supplements support kidney health without causing harm or exacerbating the condition.
Choosing the right form of B vitamins: Methylated vs. non-methylated
Selecting the right form of B vitamins, particularly in the context of chronic kidney disease (CKD), can significantly impact their effectiveness and the overall health of the individual. The choice between methylated and non-methylated B vitamins hinges on several factors, including genetic makeup and specific bodily needs.
What is methylation?
Methylation is a crucial biochemical process involving the transfer of a methyl group (one carbon atom and three hydrogen atoms) from one substance to another. This process is vital for various bodily functions, including detoxification, neurotransmitter production, eye and liver health, histamine and estrogen metabolism, and fat metabolism.
B vitamins play a significant role in this process. For instance, 5-MTHF (methylfolate) is necessary for methylation, and approximately 40% of the population in the US has a genetic variation affecting the MTHFR gene that hinders the efficient production of 5-MTHF. This inefficiency can lead to various health issues, including hormonal imbalances, cardiovascular disease, autoimmune conditions, memory issues, fatigue, allergies, poor bile production, inflammatory conditions, and depression and anxiety.
Methylated B vitamins
Methylated B vitamins, such as methylcobalamin (B12) and 5-MTHF, are more bioavailable compared to their non-methylated counterparts. This means they are readily available for the body to use and do not require conversion into their active forms. This aspect is particularly beneficial for individuals with genetic mutations that impair their ability to convert non-methylated B vitamins.
Methylated B vitamins bypass the need for several enzymatic reactions required for the conversion of non-methylated forms, making them more effective and suitable for individuals with such genetic mutations.
Moreover, studies have suggested that methylated forms, like methylcobalamin, may be more effective than other forms, such as cyanocobalamin, at improving neurological symptoms in individuals with B12 deficiency. Similarly, 5-MTHF has been found to be more effective than folic acid in reducing the risk of neural tube defects in pregnant women.
Risk of over-methylation
Some Functional medicine providers talk about the concept of over-methylation. Over-methylation is a less common condition compared to undermethylation. It occurs when there's an excess of methyl groups in the body. It is thought to be associated with elevated levels of certain neurotransmitters like dopamine, norepinephrine, and epinephrine, which are associated with a range of symptoms, including anxiety, depression, ADHD, behavior disorders, sleep disorders, restlessness, histamine intolerance, and sensitivity to environmental toxins. It is understandable that over-methylation can occur with activating mutations in the MTHFR gene. However, I could not find supportive peer-reviewed evidence for this.
Methylated vs. non-methylated B vitamins
For individuals, especially those with CKD, the choice of methylated versus non-methylated B vitamins should be made in consultation with an Integrative healthcare provider. This decision should consider individual genetic profiles, specific health conditions, and the overall dietary pattern to ensure optimal health and effective management of CKD.
The bottom line
In conclusion, B vitamins play a crucial role in supporting kidney function, particularly for individuals with Chronic Kidney Disease (CKD). The metabolic changes and nutritional challenges posed by CKD necessitate a careful approach to vitamin supplementation. Integrating a well-balanced vitamin B complex into one's dietary regimen can be a significant step toward mitigating the adverse effects of CKD.
Integrative Kidney Medicine: Bridging Traditional and Modern Approaches for a Healthier Future
Kidney disease, a silent epidemic, affects millions worldwide, often escalating to critical conditions before being diagnosed. Amidst this growing concern, integrative kidney medicine emerges as a beacon of hope. This approach unifies the wisdom of traditional, Eastern, and Functional medicine with the advancements of contemporary pharmaceutical practices. By focusing on research-backed interventions and discarding unscientific methods, integrative kidney medicine is not just a treatment approach; it's a transformation in kidney care.
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding Integrative Kidney Medicine
What is Integrative Kidney Medicine?
Integrative kidney medicine is an innovative healthcare approach that blends various medical traditions to provide holistic kidney care. It acknowledges the strengths of traditional Eastern methods, the insights of Functional medicine, and the precision of modern nutraceuticals and pharmaceuticals, creating a comprehensive care model.
Join us to end the kidney disease epidemic
Components of Diverse Medical Practices
- Traditional Medicine: Often rooted in natural remedies and ancient wisdom, focusing on balancing the body's energies.
- Eastern Medicine: Includes practices like acupuncture and herbal treatments, emphasizing holistic patient care.
- Functional Medicine: Looks at genetic, environmental, and lifestyle factors affecting health, identifying the root cause of disease, and advocating for personalized care.
- Conventional Pharmaceutical Medicine: Relies on modern drugs and technology, focusing on symptom treatment and disease management.
The Objective of Integrative Medicine
The goal is to provide personalized, effective prevention and treatment by understanding the patient's unique health profile and the intricate nature of kidney diseases. This approach fosters a deeper doctor-person relationship, leading to more informed and individualized care.
Bridging Diverse Medical Practices
A Comparative Perspective
Each medical practice brings unique strengths to kidney care:
- Traditional and Eastern medicines offer natural, less invasive options.
- Functional medicine provides a personalized approach, considering individual health histories and lifestyles.
- Conventional medicine offers advanced diagnostic tools and treatments.
Creating Synergy
Integrative kidney medicine leverages these diverse strengths, creating a synergistic approach. For instance, acupuncture from Eastern medicine can complement pharmaceutical treatments to manage pain and side effects, while Functional medicine's personalized nutrition plans can support overall kidney health.
Research-Backed Interventions
Elevating Kidney Care Through Science
The cornerstone of our integrative kidney medicine is its reliance on scientific evidence. Treatments and practices lacking empirical support are discarded, ensuring that patients receive only the most effective, safe, and modern care.
Case Studies and Research
Highlighting recent studies and real-life case studies can illustrate the effectiveness of this integrative approach. For example, research shows how certain herbal remedies, when combined with specific pharmaceuticals, can improve kidney function or slow disease progression.
Towards a Future Free of Kidney Disease Epidemic
A Visionary Approach
Integrative kidney medicine is not just about treating kidney diseases; it's about preventing them. By combining lifestyle changes, early intervention, and personalized care, this approach aims to reduce the incidence of kidney diseases significantly.
The Role of Patients and Healthcare Providers
Both patients and healthcare providers play vital roles. Patients are encouraged to be proactive about their health, while healthcare providers must stay informed about the latest research and be open to a multidisciplinary approach.
In the medical community, there has often been a divisive "us vs. them" mentality. Conventional practitioners have criticized Functional and Traditional medicine as lacking scientific basis, while Functional and Traditional practitioners accuse conventional medicine of being overly influenced by the pharmaceutical industry. To overcome this divide, we advocate for a unified approach that incorporates scientifically supported methods from all these fields, aiming to achieve our shared objective of advancing healthcare.
The Bottom Line
Integrative kidney medicine represents a paradigm shift in how we approach kidney health. Bridging the gap between various medical practices and focusing on research-backed interventions promises a future where the kidney disease epidemic is a thing of the past. As this field continues to evolve, it paves the way for more effective, holistic, and personalized healthcare, not just for kidney ailments but for a wide range of health issues.
Unveiling the New Glomerulonephritis Protocol: A Holistic Approach to Kidney Health
Kidney health is a critical component of our overall well-being, and with glomerulonephritis ranking as the third leading cause of kidney disease globally, it's time to spotlight an innovative approach to treatment. Our website is proud to introduce the new Glomerulonephritis Protocol, a comprehensive pathway that addresses not just the symptoms but the root causes of autoimmune kidney diseases.
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding glomerulonephritis
Glomerulonephritis (GN) is an autoimmune condition that affects the kidneys' ability to filter waste from your blood. It's a silent assailant that often goes unnoticed until significant damage has occurred. It primarily affects the glomeruli, which are tiny blood vessels that filter the blood in the kidneys.
Join us to end the kidney disease epidemic
The shortcomings of the conventional approach
Conventional approaches for GN have often been reactive rather than proactive. They largely ignore the complex interplay of factors that contribute to these diseases. It also neglects the root causes of these disorders. Our protocol offers an alternative by embracing the multifaceted nature of GN and providing a patient-centered approach that conventional methods lack.
Embracing integrative approach
Integrative medicine combines conventional treatment with evidence-based alternative therapies, considering all aspects of lifestyle. This is the foundation of our Glomerulonephritis Protocol—a paradigm shift towards a more empathetic, holistic form of care that sees beyond the disease to the person it affects.
The autoimmune kidney and our protocol
Our protocol explains the kidney's unique vascular structure, which makes it vulnerable to long-term damage by autoimmune attacks. By addressing the autoimmune aspect of GN, we aim to preserve kidney function and prevent long-term damage.
Types and symptoms of glomerulonephritis
The protocol encompasses various types of GN, each with unique symptoms and challenges. Conventionally, glomerulonephrites can generally be classified into two categories: "primary" and "secondary." In primary GN, the kidney is the sole apparent site of autoimmune injury. Conversely, secondary GN manifests as kidney injury accompanying a systemic autoimmune or non-autoimmune disease.
Primary glomerulonephritis includes those like IgA nephropathy, Focal segmental glomerulosclerosis (FSGS), and Membranous nephropathy.
Understanding the different types of GN is crucial for accurate diagnosis and selection of the most effective treatment strategy. However, it is vital to recognize that the classification of GN types is primarily based on the pathological features observed under the microscope. While this information is valuable in describing the disease's pattern of injury, it often does not identify the underlying root cause of the specific GN.
Glomerular injury can give rise to various symptoms and signs as it disrupts the kidney's intricate filtration process. For instance, protein may appear in the urine—a condition known as proteinuria— due to leakage through the compromised capillary walls of the glomeruli. Blood may also appear in the urine (hematuria) due to ruptured glomerular capillaries.
Addressing the four roots of glomerulonephritis
The Glomerulonephritis Protocol identifies and tackles the four roots of the disease: predisposition, triggers, mediators, and inflammation. This comprehensive approach ensures that all aspects of the disease are addressed, offering a more effective treatment pathway. It focuses on preventing further injury and rebalancing the immune response while allowing medical therapy to take effect.
The bottom line
The Glomerulonephritis Protocol on our website represents a leap forward in autoimmune kidney disease management. We invite you to explore this innovative approach, which promises a brighter future for kidney health. For more information and to see how the protocol can benefit you or your loved ones, find it here.
December Research and News 2023
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the December Research and News.
Happy Holidays!
Beta-Hydroxybutyrate Levels and Kidney Function in ADPKD
In a novel study published by Knol et al., researchers explored the relationship between beta-hydroxybutyrate ketone levels and kidney function in patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD).
The study, involving 670 patients from the DIPAK cohort, found that higher endogenous plasma beta-hydroxybutyrate concentrations might be associated with a slower decline in kidney function, as indicated by the estimated glomerular filtration rate (eGFR).
Why is this important?
This finding is significant as it suggests a potential metabolic intervention for ADPKD, a condition where cystic cells predominantly depend on glucose and have limited ability to use other energy sources like ketone bodies.
Interventions that elevate beta-hydroxybutyrate concentration could potentially slow down the rate of kidney function decline in ADPKD patients. These interventions may include a modified Ketogenic diet and intermittent fasting.
Join us to end the kidney disease epidemic
Prevalence of Sleep Apnea in CKD and ESKD Patients: A Comprehensive Meta-Analysis
A systematic review and meta-analysis conducted by Pisano et al. have brought to light the prevalence of sleep apnea (SA) in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD).
This extensive study, which synthesized data from 32 individual studies for CKD and 91 studies for ESKD, found that SA is highly prevalent in these populations, with rates of 57% in CKD and 49% in ESKD patients.
Notably, the prevalence assessed through instrumental sleep monitoring was found to be significantly higher than that estimated using sleep questionnaires.
Why is this important?
The high prevalence of sleep apnea in CKD and ESKD patients underscores a critical aspect of patient management that often might be overlooked.
This association points towards a need for more vigilant screening and diagnosis of SA in these populations, preferably using objective diagnostic tools like polysomnography or portable sleep monitors rather than relying solely on sleep questionnaires.
Identifying and treating SA in CKD and ESKD patients could improve overall health outcomes, considering the potential impact of untreated SA on cardiovascular health and quality of life.
CKD may lead to SA through a variety of mechanisms, including alterations in chemoreflex responsiveness, pharyngeal narrowing due to fluid overload, and accumulation of uremic toxins. It is also being increasingly recognized that OSA can also accelerate the loss of kidney function.
Phosphate Wasting as a Predictor of Disease Progression in ADPKD
A recent study by Xue et al. in the DIPAK cohort has unveiled significant findings regarding phosphate metabolism in Autosomal Dominant Polycystic Kidney Disease (ADPKD).
Analyzing 604 ADPKD patients, the study observed that a considerable proportion (59%) exhibited phosphate wasting. This condition was associated with various factors, including male sex, eGFR, levels of fibroblast growth factor-23 (FGF-23), parathyroid hormone (PTH), and Copeptin.
More crucially, phosphate wasting correlated with a more rapid decline in eGFR and increased the risk of adverse kidney outcomes.
Why is this important?
This research provides valuable insights into the importance of phosphate metabolism in ADPKD, a disease typically characterized by cyst formation and kidney enlargement.
The association of phosphate wasting with accelerated disease progression in ADPKD indicates that it could serve as a potential biomarker for disease prognosis.
In fact, phosphate wasting, according to this study, can be an early sign of tubular dysfunction in patients with ADPKD. It can also lead to inflammation and faster progression of kidney disease.
Iron Levels and Cardiovascular Risk in CKD
In a significant study published by Hasegawa et al., researchers investigated the association between serum iron markers and cardiovascular disease (CVD) risk in patients with pre-dialysis chronic kidney disease (CKD).
The study, which included 1,416 CKD patients, examined serum transferrin saturation and ferritin levels in relation to the risk of CVD events.
Notably, it found that patients with serum transferrin saturation below 20% faced a higher risk of CVD and congestive heart failure.
Furthermore, iron supplementation was shown to be more beneficial in reducing CVD risk among patients with lower transferrin saturations.
Why is this important?
This study sheds light on the crucial link between iron metabolism and cardiovascular health in CKD patients. The findings suggest that monitoring and maintaining adequate serum transferrin saturation levels could be a key strategy in reducing the risk of cardiovascular events, a major cause of morbidity and mortality in CKD.
The effectiveness of iron supplementation in lowering CVD risk, especially in those with lower transferrin saturation, highlights the need for personalized treatment approaches in managing CKD patients.
It is, therefore, important to correct iron levels in CKD patients even if they do not have anemia.
Review article of the month
Diet and Polycystic Kidney Disease
Polycystic Kidney Disease (PKD) is a hereditary condition characterized by the growth of cysts in the kidneys and other organs. This chronic and progressive disease has recently seen a growing interest in the potential of dietary modifications as a means to prevent or slow its progression.
While vasopressin-receptor agonists have been a novel pharmacological breakthrough, recent trials and clinical studies have also shed light on dietary-related therapeutic strategies.
This review compiles the current evidence on the impact of nutrients, foods, and dietary patterns on the growth and progression of PKD cysts.
It also discusses the existing evidence-based dietary care for PKD patients and outlines the potential for further development of evidence-based dietary interventions, highlighting their possible implications in managing and treating PKD.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
An Integrative Approach for Stage 4 Kidney Disease: Balancing Diet, Oxidative Stress, and Conventional Treatments
Chronic kidney disease (CKD) is a progressive condition marked by a gradual loss of kidney function over time. By the time a patient reaches stage 4, the penultimate stage of CKD, the importance of an integrative approach to management cannot be overstated. This approach not only involves careful attention to diet and lifestyle but also the incorporation of conventional medications to manage symptoms and slow progression. In this blog, we will discuss the various aspects of an integrative approach for stage 4 kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
Dietary considerations: Navigating acid Load and potassium
One of the critical aspects of managing stage IV kidney disease is dietary modification. Diet in CKD is nuanced and requires a balance between restricting certain elements while ensuring adequate nutrition. A key concern is the dietary acid load. High acid diets, often rich in animal proteins, can exacerbate kidney burden, hastening the decline in renal function. Therefore, a shift towards a plant-dominant diet, lower in acid load, can be beneficial.
However, this brings us to the conundrum of potassium. Many plant-based foods are rich in potassium, which in the context of CKD can accumulate in the blood and cause hyperkalemia, a potentially life-threatening condition. Thus, patients and caregivers must navigate the tightrope of reducing dietary acid load while managing potassium intake.
It's crucial to understand that adopting an alkaline, plant-dominant diet to reduce dietary acid load may also influence potassium levels in the bloodstream. Historical research, including a study from the 1950s, indicated that for every 0.1 unit decrease in extracellular pH, plasma potassium concentration could rise by 0.6 mEq/L. Simply put, an increased dietary acid load and higher blood acidity correlate with elevated potassium levels in the blood. In contrast, a lower acid load from an alkaline diet could help manage potassium levels.
Nevertheless, when managing potassium levels remains a challenge, it is essential to carefully choose plant-based foods with lower potassium content. Options such as apples, berries, asparagus, broccoli, cabbage, cauliflower, celery, and corn, for example, can be safely incorporated into the diet. Additionally, employing specific cooking methods can significantly reduce the potassium in foods that typically have high amounts; for instance, leaching vegetables through a process like double boiling can effectively lower their potassium content, making them more suitable for patients with such dietary restrictions.
Join us to end the kidney disease epidemic
Phosphorus: The early bird of dietary restrictions
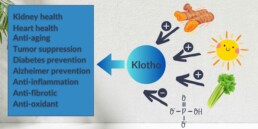
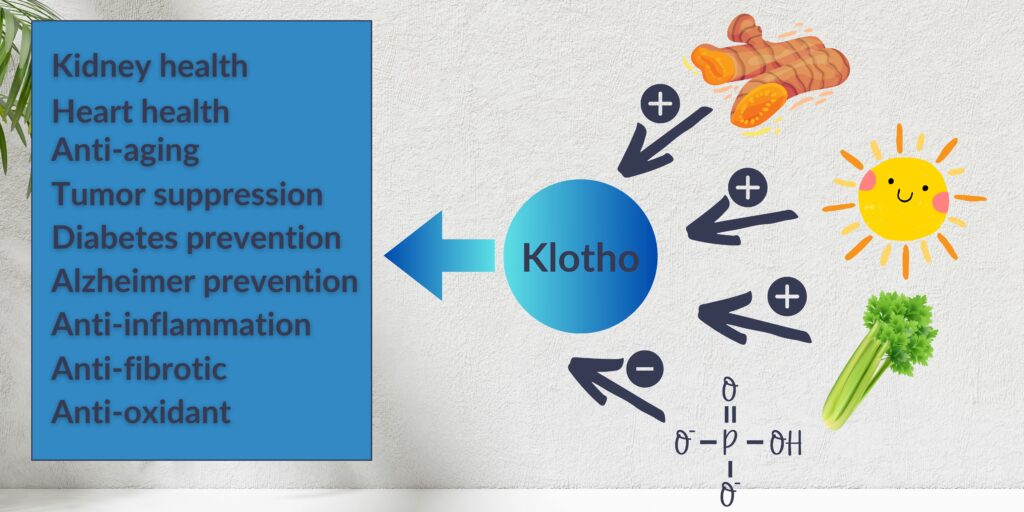
Phosphorus restriction is another facet of the dietary approach in CKD that needs attention well before stage IV. Phosphate, which is commonly described as phosphorus, is abundant in foods like dairy products, nuts, seeds, whole grains, animal proteins, and processed foods through additives. Higher intake of phosphorus can lead to the activation of the FGF23-Klotho axis. The latter can lead to bone and cardiovascular issues. This makes controlling phosphorus intake vital for all patients with CKD.
An integrative approach involves choosing foods with lower phosphorus bioavailability, such as plant-based sources, since the phosphorus in these foods is less absorbable compared to animal-based sources. Moreover, it's essential to be vigilant about food labels to avoid phosphorus additives in processed foods.
The antioxidant powerhouse: Supporting glutathione
High levels of oxidative stress and inflammation often accompany Stage IV CKD. Glutathione, a potent antioxidant, is crucial in mitigating these harmful processes. However, in kidney disease, higher oxidative stress can deplete the body's glutathione.
To bolster glutathione levels, one strategy is to enhance the intake of its precursor amino acids, such as cysteine, glycine, and N-acetyl cysteine. This can be achieved through a balanced diet that includes moderate amounts of sulfur-rich proteins—found in meats like beef, fish, and poultry—and a variety of sulfur-containing vegetables, particularly those from the allium family (such as garlic, onions, leeks, and chives) and cruciferous vegetables like broccoli and kale.
Maintaining this balance is key and can be effectively managed with a predominantly plant-based diet that judiciously includes, rather than entirely excludes animal proteins. This approach ensures adequate provision of essential nutrients while supporting kidney health.
Preventing fibrosis and supporting mitochondria
Renal fibrosis, the final common pathway in CKD leading to end-stage renal disease, is a key therapeutic target. To prevent fibrosis, it's critical to maintain a balanced diet and manage blood pressure and blood sugar levels. Certain nutrients, such as resveratrol found in grapes and berries, may have antifibrotic properties, while omega-3 fatty acids can help reduce inflammation, contributing to fibrosis.
Energy utilization and mitochondrial support are also crucial. Mitochondrial dysfunction is a contributor to CKD progression. Coenzyme Q10 and L-carnitine are nutrients that support mitochondrial function and may be considered for supplementation. While we often recommend Coenzyme Q10 to these patients, we do not recommend the use of oral L-carnitine. This is because the gut bacteria can metabolize it into TMAO, which is one of the uremic toxins that are associated with poor cardiovascular outcomes. It is, therefore, important to have a healthcare professional guide this supplementation, as CKD affects the metabolism and excretion of various compounds.
Medication and supplement dosing: The fine tuning
Conventional medications play a critical role in the management of stage IV CKD. Angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), and sodium-glucose cotransporter 2 (SGLT2) inhibitors are cornerstones in conventional medicine approach to slowing the progression of kidney disease and managing hypertension, a common comorbidity.
However, as kidney function declines, dosing adjustments for medications and supplements are often required to prevent toxicity. It's important for healthcare providers to regularly review and adjust medication regimens as the disease progresses.
Moreover, some supplements may be contraindicated or require dosing adjustments in CKD. For example, magnesium and certain fat-soluble vitamins can accumulate to harmful levels if not properly monitored. Therefore, an integrative approach to CKD must include a close partnership with a nephrologist to ensure the safe and effective use of medications and supplements.
The bottom line
Managing stage IV CKD requires an integrative approach that melds dietary management, the support of antioxidant systems, conventional pharmacotherapy, and lifestyle changes. This approach addresses the multifaceted challenges of CKD, aiming not only to slow disease progression but also to improve quality of life. With thoughtful dietary choices that balance the reduction of dietary acid load and phosphorus with careful potassium intake, alongside antioxidant support, fibrosis prevention, and energy optimization, patients can tackle CKD from several angles.
It's imperative for patients and healthcare professionals to work closely together, continually adjusting treatment plans as the disease evolves. Through this collaborative, nuanced approach, the burdens of stage IV CKD can be mitigated, offering patients a semblance of control and hope as they navigate this complex chronic disease.
Smoking and Kidney Disease Progression Globally and in the US
Kidney disease remains a silent threat affecting millions worldwide, with the United States recording significant numbers in its grip. The link between smoking and kidney disease progression is well-established, posing an additional risk to those with lifestyle-related or genetic predispositions to renal ailments. Understanding this link is crucial for prevention strategies and health education.
By Majd Isreb, MD, FACP, FASN, IFMCP
Kidney disease: A silent global challenge
Kidneys, the sophisticated filtration system of the body, are vital to maintaining overall health. When chronic kidney disease (CKD) strikes, it often does so silently, gradually diminishing the kidneys' ability to cleanse the blood of toxins. Globally, CKD is a rising health concern, with a prevalence that suggests an urgent need for widespread public health initiatives and lifestyle modifications.
The Correlation Between Smoking and Rising Kidney Disease Rates
Recent comprehensive meta-analyses provide compelling evidence that cigarette smoking stands as a potent independent risk factor for the onset of CKD. These studies shed light on the heightened vulnerability of current smokers, who face a substantially higher risk of developing CKD when compared to non-smokers. The risk escalates with the duration and intensity of smoking, indicating a cumulative effect that worsens over time.
Notably, the incidence of CKD in smokers is often seen in conjunction with other serious conditions, such as hypertension and diabetes mellitus, which are primary contributors to kidney disease worldwide. Smokers who develop CKD are, therefore, usually classified as hypertensive or diabetic kidney disease.
How smoking accelerates kidney disease: The biological backdrop
Smoking's role in the development of CKD is not limited to increasing its onset; it also significantly hastens its advancement. Research indicates that individuals with CKD who have a smoking history, even if it totals fewer than 15 pack-years, are more susceptible to an accelerated decline in kidney function. This susceptibility intensifies with prolonged smoking habits.
However, there's a silver lining: the likelihood of CKD progression diminishes progressively the longer an individual remains abstinent from smoking. Therefore, quitting smoking emerges not only as a preventive measure but also as a critical strategy for slowing the progression of CKD, providing hope for improved outcomes in former smokers.
The conversation around smoking and kidney disease progression is, therefore, critical. Smoking introduces toxins that have a direct impact on renal function. These toxins trigger oxidative stress and damage the vascular endothelium, the inner lining of blood vessels. As a consequence, blood vessels may narrow, leading to diminished circulation to the kidney. This restriction can intensify issues such as hypertension and inflammation, both of which are critical contributors to the onset and progression of CKD.
The role of cadmium exposure from smoking in kidney disease progression
Cadmium, a heavy metal found in cigarette smoke, is another insidious factor in the narrative of smoking and kidney disease progression. This toxic metal accumulates in the kidneys, where it can cause significant damage to the renal tubules, leading to a decrease in their filtering capacity. Long-term exposure to cadmium from smoking not only increases the risk of developing kidney disease but also exacerbates existing conditions, making it a double threat.
Studies have shown that each cigarette exposes the smoker to 0.5-1 microgram of cadmium. Lead has also been found in cigarettes. High blood levels of lead were found in smokers as compared to non-smokers.
Interplay between sleep patterns, smoking habits, and kidney health
Recent findings from the National Health and Nutrition Examination Survey (NHANES) 2017–2018 shed light on the intricate dynamics between sleep, smoking, and kidney function. The study, which classified participants into subgroups based on their smoking frequency and sleep duration (short, normal or long), revealed that individuals with shorter sleep spans exhibited higher levels of serum cotinine, a marker of nicotine intake
Non-smokers with either excessively short or long sleep durations had lower estimated glomerular filtration rates (eGFR) — a key indicator of kidney function — compared to those who smoked every day and slept less than 6 hours. The relationship between sleep duration and kidney function varied based on smoking status. Interestingly, habitual smokers experienced an increase in eGFR with reduced sleep, whereas occasional smokers showed improved kidney function with longer sleep.
Notably, for non-smokers, a normal sleep duration not only correlated with optimal eGFR levels, displaying a U-shaped relationship, but also emerged as a significant protective factor for kidney health, underscoring the importance of balanced sleep, particularly for individuals who do not smoke.
Join us to end the kidney disease epidemic
Analyzing the incidence of kidney disease in the US
In the United States, the incidence of kidney disease has been a growing concern, with current estimates indicating that over 37 million Americans are affected. This number is not merely a statistic; it represents a widespread public health issue with profound personal and economic impacts. The role of smoking in this is significant, with smokers having a higher likelihood of albuminuria, an early marker of CKD.
Smoking and kidney disease progression: A preventable risk factor
Smoking and kidney disease progression is a modifiable risk factor. Smoking cessation is a powerful intervention, with evidence showing that quitting can slow down the progression of CKD, especially in the early stages. Public health campaigns and clinical interventions often focus on smoking cessation as a critical component of kidney disease management.
The global picture: Smoking and kidney disease incidence
Worldwide, the incidence of kidney disease varies, influenced by factors such as genetics, healthcare infrastructure, and lifestyle choices, including smoking. The global health community recognizes the necessity of addressing smoking as part of the strategy to reduce the burden of CKD. Initiatives like World Kidney Day underscore the importance of lifestyle choices on kidney health.
The way forward: Combating smoking to protect kidney health
To disrupt the trajectory of smoking and kidney disease progression, a multifaceted approach is essential. This includes policy-driven actions like smoking bans, taxation, and education campaigns, alongside clinical strategies such as screening programs and personalized interventions for those at risk.
The bottom line
In conclusion, the intertwining of smoking and the progression of kidney disease presents a clear message: prevention is possible. Tackling smoking habits can play a pivotal role in reducing the incidence of kidney disease both in the U.S. and around the world. As healthcare providers, policymakers, and individuals, the collective effort to diminish the impact of smoking on renal health is not only necessary but imperative for a healthier global population.
November Research and News 2023
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the November Research and News.
Impact of Resveratrol on Endothelial Function in CKD and Diabetes Patients
In a randomized controlled trial by Gimblet et al., the effects of resveratrol, a cardioprotective compound, were tested on patients with both chronic kidney disease (CKD) and diabetes—a group particularly vulnerable to cardiovascular diseases due to impaired endothelial function.
The study's findings were optimistic, showing a significant improvement in endothelial function after a 6-week period of supplementation with 400 mg/day of resveratrol, as measured by brachial artery flow-mediated dilation.
Why is this important?
The endothelium plays a vital role in vascular health, and its dysfunction is a precursor to atherosclerosis.
The improvement seen with resveratrol suggests that it might be a viable supplement to improve vascular health in CKD patients with diabetes without altering other cardiovascular risk factors such as blood pressure or HbA1c levels.
This could pave the way for a new adjunct therapy that targets a significant risk factor for mortality in this patient population, potentially improving clinical outcomes and quality of life.
Urinary Oxalate Levels as a Predictor for CKD Risk
A large-scale, longitudinal observational study by Puurunen et al. has identified 24-hour urine oxalate (UOx) excretion as a potential risk factor for incident chronic kidney disease (CKD).
Analyzing data from over 426,000 individuals without CKD at baseline, the study found that higher levels of UOx were associated with an increased risk of developing CKD, with an even greater risk observed among individuals with malabsorptive conditions.
Why is this important?
Understanding the risk factors for CKD is crucial for early intervention and prevention. This study suggests that UOx could be a modifiable risk factor, which, if addressed, might help to reduce the incidence of CKD.
The implications are significant for both patient lifestyle modifications and potential clinical interventions. For patients with elevated UOx levels, particularly those with malabsorptive conditions, monitoring, and possible dietary modifications could become part of CKD preventative strategies.
Join us to end the kidney disease epidemic
Modulating Retinoic Acid Signaling for Acute Kidney Injury Protection
Yang et al. have made a significant discovery that reactivation of RAR signaling occurs in proximal tubular epithelial cells (PTECs) during acute kidney injury (AKI).
By genetically inhibiting RAR signaling in these cells, the study demonstrates protection against AKI in experimental models. Interestingly, this inhibition is linked to increased PTEC injury markers such as Kim1, which paradoxically contributes to beneficial effects through enhanced apoptotic cell clearance and subsequent cellular repair processes.
Why is this important?
Acute kidney injury is a critical concern for patients with CKD, as it can exacerbate the progression of the disease.
This research provides insight into a counterintuitive approach where inhibiting a pathway often associated with development can protect adult kidney cells from injury.
The findings open up new avenues for therapeutic interventions in AKI and possibly CKD, focusing on the modulation of RAR signaling and the enhancement of the body's natural repair mechanisms.
This approach could potentially alter the treatment landscape for patients at risk of AKI due to sepsis or other causes.
Exercise as a Modulator of Kidney Biomarkers in Older Adults
The LIFE trial, as explored by Sheshadri et al., delved into whether a structured moderate-intensity exercise program could affect biomarkers indicative of kidney health in sedentary older individuals.
Over 1,381 participants aged 70-89 were assessed through a randomized controlled trial contrasting exercise with health education over two years.
Why is this important?
While randomization to the exercise program did not uniformly improve kidney biomarkers across the group, higher physical activity levels were positively correlated with improvements in specific biomarkers associated with glomerular injury, tubular function, and repair.
This suggests a more nuanced relationship between exercise and kidney health, where individual increases in activity can lead to tangible benefits for kidney function.
These findings underscore the potential for structured physical activity to serve as a non-pharmacological strategy to combat kidney health deterioration in the aging population.
Such insights are particularly relevant for preventive health strategies in CKD, where maintaining kidney function is critical.
Review Article of the Month
Oxidative stress and the role of redox signaling in chronic kidney disease
The review article published in Nature Reviews Nephrology examines the complex role of oxidative stress and redox signaling in the progression of chronic kidney disease (CKD).
Reactive oxygen species (ROS), while essential at low levels for various cellular functions such as signal transduction and immune responses, can be detrimental in excess.
High levels of ROS are implicated in the development and exacerbation of CKD. Attempts to use antioxidants to curtail ROS and combat CKD have been largely unsuccessful, pointing to the need for a more refined approach.
Recent advancements in understanding the specific molecular interactions of ROS and their targets suggest the possibility of developing targeted therapies. These therapies would aim to selectively modulate ROS-mediated pathways, offering a more sophisticated and potentially effective approach to treating CKD.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Embracing Holistic Hypertension Management: A Comprehensive Guide
High blood pressure, also known as hypertension, is a silent yet potent threat to millions worldwide. It's a condition that often requires a multifaceted approach to manage effectively. "Holistic hypertension management" is not just a buzzword; it's a comprehensive method that encompasses diet, lifestyle, and beyond. This guide explores how an integrative strategy can combat hypertension, taking into account the intricate roles of genetics and the gut microbiome.
By Majd Isreb, MD, FACP, FASN, IFMCP
Hypertension means the blood pressure is higher than normal. Blood pressure is how hard the blood pushes against the walls of the arteries, which are the tubes that carry blood away from the heart to the rest of the body.
It's measured in millimeters of mercury (mmHg) and given as two numbers. The first (systolic) number is the pressure when the heart beats, and the second (diastolic) number is the pressure when the heart rests between beats.
Normal blood pressure is around 120/80 mmHg. It's high if it's 130/80 mmHg or more. Often, there are no clear symptoms, but high blood pressure can lead to serious health issues like heart disease and strokes.
All medical guidelines for the management of hypertension recommend starting with “lifestyle modifications.” However, the details of these modifications are often omitted. So, let’s discuss these in some detail.
Join us to end the kidney disease epidemic
The Genetic Perspective
Genetics can predispose individuals to hypertension, making some more susceptible than others. More than 100 genetic variants have been linked to essential hypertension. Yet no conclusive candidate gene have been found to date. There are, however, clear genetic disorders that can cause secondary forms of hypertension, such as Liddle syndrome and familial hyperaldosteronism.
Understanding one's genetic makeup can guide personalized treatment plans. Although we can't alter our genetics, recognizing the genetic component can inform a more tailored holistic hypertension management plan.
Dietary Changes for Hypertension
Diet is the cornerstone of managing high blood pressure. The DASH (Dietary Approaches to Stop Hypertension) diet and the Mediterranean diet are celebrated for their effectiveness in lowering blood pressure naturally. These diets are rich in fruits, vegetables, whole grains, and healthy fats like olive oil.
Reducing sodium intake is also crucial, as excess salt is a known contributor to elevated blood pressure. Foods rich in potassium, magnesium, and calcium have a harmonizing effect on blood pressure levels, making them vital components of holistic hypertension management.
Finally, regular consumption of cocoa and moderate red wine, as part of a balanced diet, may confer anti-hypertensive benefits. Including fiber-rich foods and flax seeds can improve blood pressure control. Omega-3 fatty acids from fish or supplements have been shown to lower blood pressure, as have inorganic nitrates found in leafy greens and beets.
Lifestyle Modifications
Lifestyle plays an equally important role in controlling hypertension. Both aerobic and resistance exercises have been found to improve blood pressure control. Regular physical activity, whether it's brisk walking, cycling, or swimming, can significantly lower blood pressure.
In addition, stress-reduction techniques such as transcendental meditation, deep breathing exercises, and yoga are not only beneficial for mental health but have also been shown to reduce blood pressure.
Lastly, adequate sleep and cessation of smoking and alcohol are imperative in the holistic management of hypertension.
Supplements and Herbs
In the realm of holistic hypertension management, certain herbs and supplements can be supportive allies. Magnesium, vitamin D, coenzyme Q10, and omega-3 fatty acids have all been linked to blood pressure reduction. Herbal remedies like garlic, hawthorn, and olive leaf extract may also offer benefits. However, it's essential to consult with an Integrative healthcare professional before starting any new supplement or herb to ensure they're appropriate and safe.
Gut Microbiome and Blood Pressure
Recent evidence underscores the gut microbiome's critical role in regulating blood pressure, moving from mere association to proven causation. Gut microbes affect blood pressure by altering gene pathways in the host, with some microbial metabolites being beneficial, like short-chain fatty acids, while others like trimethylamine N-oxide can be harmful.
Dysbiosis, or microbial imbalance, can weaken the gut barrier, leading to inflammation and influencing blood pressure control systems like the renin-angiotensin-aldosterone system. Addressing these microbiome-related factors is becoming an integral part of holistic hypertension management, aiming to harness the microbiome's potential to improve cardiovascular health through diet, probiotics, and precision medicine.
Addressing sleep apnea
Obstructive sleep apnea (OSA) is closely linked with the development of hypertension. This sleep disorder, characterized by pauses in breathing during sleep, is found in more than half of those with high blood pressure. Research indicates that individuals with OSA are at a greater risk of developing high blood pressure, a concern especially notable in children with OSA, who are three times more likely to become hypertensive in their teen years.
The relationship between OSA and hypertension is so strong that OSA is considered a major modifiable risk factor for high blood pressure, which, in turn, significantly increases the risk of heart disease. Addressing OSA is essential in hypertensive patients to mitigate this risk. Treatments like CPAP therapy have been shown to reduce the blood pressure spikes associated with OSA, underscoring the need for early detection and management of sleep apnea in the context of hypertension.
The bottom line
High blood pressure is a complex condition, but it can be managed effectively with a holistic approach. By integrating diet, lifestyle changes, and an understanding of one’s genetics and gut health, "Holistic Hypertension Management" can lead to improved blood pressure control and overall health.
The Shadowed Link: Unveiling How Drugs of Abuse and Kidney Health Are Connected
The nexus between drugs of abuse and kidney health remains a shadowed strand in the broader web of public health concerns. As the prevalence of drug abuse escalates, the consequential impact on kidneys emerges with increasing clarity. This connection is intricate and profound, with various substances clandestinely inflicting damage on renal functions.
By Majd Isreb, MD, FACP, FASN, IFMCP
The spectrum of drugs of abuse that can affect the kidneys ranges from synthetic cannabinoids to potent stimulants and unveils a series of renal pathologies that often go unnoticed until critical complications arise. Understanding the enigmatic relationship between drugs of abuse and kidney health is imperative in the quest for proactive healthcare measures, timely diagnosis, and effective treatment strategies.
Synthetic cannabis and kidney health: A deceptive duo
Synthetic cannabis, often masked under names like spice or K2, has a deceptive potency that belies its visual similarity to marijuana. Despite their benign appearance, these substances are far from harmless, especially concerning kidney health.
Individuals ingesting these drugs can present with symptoms mimicking more mundane conditions—nausea, vomiting, and abdominal pain—yet what transpires at a renal level is anything but benign. Toxic acute tubular necrosis (ATN) often ensues, a direct hit to the kidneys' filtration system.
The diagnosis is challenging due to varied urinalysis results and the absence of detection in routine drug screenings, making a keen clinical awareness crucial for identification.
Join us to end the kidney disease epidemic
The hidden hazards of bath salts on renal functions
"Bath salts," a street name for synthetic cathinones, are a class of drugs that masquerade as harmless yet wield devastating effects on kidney health. These psychoactive substances induce a perilous state of heightened adrenergic activity, manifesting as altered mental states and extreme physical symptoms such as tachycardia (rapid heart rate) and hypertension (high blood pressure). When bath salts abuse culminates in acute kidney injury (AKI), the usual suspect is rhabdomyolysis (muscle breakdown); however, AKI can occur without this muscle injury, making the connection to kidney health all the more insidious.
MDMA's impact on the kidneys: A hidden euphoric toll
3-4 Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy, is often associated with the pursuit of euphoria and heightened sensory perception. However, the increase in serotonin, dopamine, and noradrenaline levels can also lead to a dangerous increase in thirst and subsequent acute hyponatremia—a condition where sodium levels in the blood become dangerously low. This condition is due to inappropriate secretion of arginine vasopressin and seems to affect women more frequently than men.
While MDMA-related AKI often trails in the wake of rhabdomyolysis, cases without such muscle breakdown challenge clinicians, revealing the nuanced dangers these drugs pose to kidney health.
Ketamine abuse: An anesthetic's chronic peril to kidneys
Ketamine, with its legitimate medical uses as an analgesic and anesthetic, has a dark side when used illicitly over long periods. Chronic abuse can lead to a form of AKI, where ketamine metabolites crystallize in the urinary system, causing obstruction and damage. Kidney injury from ketamine can occur without obstruction, highlighting a complex and often overlooked threat to renal health.
The profound effects of cocaine on kidney health
Cocaine's vasoconstrictive properties (it constricts the blood vessels) are well-documented, but its effects extend far beyond the immediate high and potential for addiction. As a powerful stimulant, cocaine can lead to severe hypertension and subsequent AKI, often associated with rhabdomyolysis and ATN.
Furthermore, the use of cocaine contaminated with levamisole has been linked to ANCA-associated vasculitis, a serious condition involving small blood vessels, including those in the kidneys. These scenarios underscore cocaine's significant, albeit less discussed, impact on kidney health.
The Bottom Line
The clandestine connection between drugs of abuse and kidney health is a concern that demands attention. As healthcare providers, we must look beyond the immediate psychotropic effects of these substances and consider their insidious impact on kidney health. With synthetic cannabis, bath salts, MDMA, ketamine, and cocaine leaving an indelible mark on the kidneys, our strategies for addressing drug abuse must evolve to include renal protection measures.
To mitigate these risks, a holistic approach is required—one that encompasses not only the management of drug addiction but also the prevention and early detection of kidney injury. It is imperative that we educate both the public and healthcare professionals about the potential renal complications of drug abuse, encouraging vigilance and swift intervention.
Exploring the Role of Postbiotics in Enhancing Kidney Health
The realm of gut microbiota and its impact on overall health is an expanding frontier in medical science. Among the latest discoveries within this domain are postbiotics, a term that is becoming increasingly significant, especially in the context of chronic illnesses such as kidney disease. In this comprehensive exploration, we will delve into how postbiotics and kidney health are interconnected, shedding light on the potential for these substances to support renal function and contribute to kidney disease management.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Emergence of Postbiotics
To appreciate the value of postbiotics, it's essential to understand what they entail. As the next frontier following probiotics and prebiotics, postbiotics include a broad spectrum of bioactive compounds — from enzymes and short-chain fatty acids to cell-free supernatants and bacterial lysates. These byproducts result from the metabolic activities of probiotics and are found to exert health benefits even without the presence of live microorganisms.
Join us to end the kidney disease epidemic
Postbiotics and Kidney Health: A Synergistic Relationship
The kidneys are the body's natural filtration system, playing a crucial role in waste elimination, fluid balance, and electrolyte management. Chronic kidney disease (CKD) represents a growing health concern, with numerous studies linking gut health to renal function. Postbiotics come into play as potential therapeutic agents that can positively influence kidney health through several mechanisms.
The Anti-Inflammatory Potential of Postbiotics
Chronic inflammation is a known perpetrator in the progression of kidney disease. Postbiotics have been shown to possess anti-inflammatory properties, which could make them beneficial in managing CKD. By mitigating inflammatory pathways, postbiotics may help slow down renal impairment and improve the quality of life for those with kidney disease.
Antioxidant Properties and Their Implications
Oxidative stress is another contributing factor to kidney damage. Postbiotics, through their antioxidant activities, have the potential to neutralize free radicals, thus protecting the kidneys from oxidative damage. This antioxidative capacity could be crucial in developing new supportive therapies for CKD patients.
Gut-Kidney Axis: The Target of Postbiotic Action
The gut-kidney axis is an emerging concept highlighting how gut health can reflect upon kidney function. Uremic toxins produced in the gut can have deleterious effects on the kidneys. By modulating the gut microbiota and reducing the production of these toxins, postbiotics could alleviate some of the burdens on the kidneys, offering a novel approach to managing kidney health. They can be used as a part of a comprehensive gut restoration protocol.
Clinical Insights: Postbiotics as a Therapeutic Option
While research is ongoing, some clinical studies have already indicated that postbiotics can be beneficial for patients with kidney disease. These findings suggest a promising role for postbiotics in clinical settings, advocating for their inclusion in kidney disease management protocols. Postbiotics may also have a significant role in the prevention of kidney stones.
Incorporating Postbiotics into a Kidney Health Diet
As we continue to understand the impact of postbiotics, dietary strategies for kidney health could evolve to include these compounds. Whether through supplementation or the consumption of foods rich in postbiotics, there is a clear pathway for these bioactive compounds to support kidney health.
To enhance kidney function, incorporating fermented foods rich in postbiotics into the diet can be beneficial. Foods like kefir, tempeh, and kimchi are not only nutritious but also contribute to a balanced gut microbiota, which is closely linked to kidney health. By prioritizing a diet that fosters healthy gut bacteria, one can take a proactive step toward maintaining optimal kidney function and overall well-being.
The Future of Postbiotics in Kidney Health Management
As the scientific community delves deeper into the potential health benefits of postbiotics, we may soon witness a paradigm shift in how kidney diseases are managed. Integrating postbiotics into treatment regimens holds the promise of a more holistic approach to combating CKD and improving patient outcomes.
The bottom line
Postbiotics and kidney health are linked in a way that offers hope for those battling CKD. With their anti-inflammatory and antioxidant effects, along with their ability to modulate the gut-kidney axis, postbiotics represent a burgeoning field of study with the potential to revolutionize kidney health strategies. As research progresses, the integration of postbiotics into the management of kidney diseases seems not just plausible but inevitable, paving the way for groundbreaking treatments that harness the power of these remarkable compounds.
Exploring the Glycocalyx: A Key Player in Kidney Health and Pathology
Understanding the true depth and potential of the human body requires delving into the intricacies of its smallest components. The glycocalyx, a sugar-coated web enveloping cells, is one such component that plays an essential role in various bodily functions. This article will explore the fascinating world of glycocalyx, its relevance to kidney health, and the implications of its dysfunction in kidney diseases.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Glycocalyx: An overview
The glycocalyx, a highly complex structure, forms a protective, gel-like layer over the surfaces of cells. It is primarily composed of glycoproteins, glycolipids, and proteoglycans. This structure serves as the cell's interface with its environment, mediating cellular interactions, protecting against physical and chemical stress, and playing a key role in cell recognition and signaling processes.
Physiological factors that affect glycocalyx
A healthy glycocalyx is essential for various biological functions, so understanding physiological factors that can harm it is vital for preventing and treating a wide range of diseases. Several substances and physiological factors can disrupt the integrity and function of the glycocalyx:
- Enzymatic Degradation: Certain enzymes can degrade the components of the glycocalyx. For example, heparanase can degrade heparan sulfate, one of the primary constituents of the glycocalyx.
- Inflammatory Mediators: Inflammatory mediators, such as cytokines and chemokines, can disrupt the glycocalyx. During inflammation, these substances can lead to the release of enzymes that degrade the glycocalyx.
- Oxidative Stress: Oxidative stress, caused by an imbalance of reactive oxygen species (ROS) and the body's ability to counteract their harmful effects, can damage the glycocalyx.
- Hyperglycemia: High blood sugar levels, as seen in diabetes, can lead to glycocalyx damage. Prolonged hyperglycemia can result in structural changes to the glycocalyx, making it less effective at performing its functions.
- Ischemia and Reperfusion: The process of temporarily cutting off the blood supply (ischemia) and subsequently restoring it (reperfusion) can cause significant harm to the glycocalyx. This is often seen in certain surgical procedures or conditions like stroke or heart attack.
- Mechanical Forces: High levels of shear stress or pressure on the blood vessel walls can also lead to disruption and shedding of the glycocalyx.
Join us to end the kidney disease epidemic
Uric acid and glycocalyx
Elevated uric acid levels have also been associated with disruption of the endothelial glycocalyx. Uric acid is a waste product normally filtered out by the kidneys, and high levels in the blood, a condition known as hyperuricemia, can occur due to various factors like diet, genetics, or impaired kidney function.
Studies suggest that hyperuricemia may lead to oxidative stress and inflammation, which, in turn, can contribute to damage and shedding of the endothelial glycocalyx. This could play a role in developing diseases like hypertension and cardiovascular disease. Hyperuricemia has been linked to faster progression of kidney disease via glycocalyx shedding.
It's worth noting that while uric acid can indeed be harmful at high levels, it also has an antioxidant role at physiological concentrations. Therefore, the concentration of uric acid and the individual's overall metabolic context are important factors to consider in its impact on the glycocalyx.
The glycocalyx and kidney function
The kidney, a crucial organ in the human body, relies on the glycocalyx to function effectively. One of the kidney's main jobs is filtration, a task performed by specialized structures known as glomeruli. Each glomerulus is made up of a network of tiny blood vessels, or capillaries, which are lined with a thin layer of the glycocalyx.
The glycocalyx of the glomerular endothelial cells plays a key role in maintaining the selective permeability of the glomerular filtration barrier (GFB), a critical factor in kidney function. This barrier allows water and small molecules to pass into the urine while retaining larger molecules like proteins within the bloodstream. The glycocalyx's unique structure and composition enable this selectivity, thereby playing a pivotal role in maintaining the body's fluid and electrolyte balance.
The glycocalyx in kidney disease
Damage or loss of the glycocalyx can have profound implications for kidney health, leading to a range of conditions collectively referred to as glomerular diseases. When the glycocalyx is compromised, the GFB's selectivity is affected, leading to proteinuria, a condition characterized by an excess of proteins in the urine, a common sign of kidney disease.
Research indicates that the glycocalyx's degradation is associated with diabetes, hypertension, and chronic kidney disease (CKD). In particular, the progression of CKD involves ongoing damage to the glycocalyx, promoting inflammation and fibrosis, eventually leading to end-stage kidney disease.
Addressing glycocalyx damage: The way forward
Understanding the critical role of glycocalyx in kidney health has opened new avenues for treating kidney diseases. Therapies targeting the preservation and restoration of the glycocalyx hold promise in managing kidney diseases more effectively. For instance, certain medications like sulodexide, a highly purified glycosaminoglycan, have shown potential in clinical trials to restore the glycocalyx and reduce proteinuria.
Nurturing the glycocalyx through comprehensive lifestyle strategies
An integrative or holistic approach to improving or healing the glycocalyx would consider the entire body and lifestyle, emphasizing a balance between physical health, mental well-being, and environmental influences. Here are some strategies that could potentially support glycocalyx health:
- Balanced Diet: Consuming a diet rich in antioxidants, such as fresh fruits, vegetables, and whole grains, can help combat oxidative stress, one of the factors that can damage the glycocalyx. Also, minimizing sugar and processed food intake may prevent advanced glycation end-products that harm the glycocalyx.
- Regular Exercise: Moderate and consistent exercise can enhance blood flow, encouraging the health and regeneration of the vascular system, including the glycocalyx.
- Stress Management: Chronic stress releases hormones that can contribute to inflammation and potential glycocalyx degradation. Practices like meditation, yoga, or other stress-reducing activities could be beneficial.
- Adequate Hydration: Proper hydration is crucial for maintaining blood flow and kidney function, helping effectively remove toxins that could otherwise harm the glycocalyx.
- Supplements: Certain supplements, such as those containing antioxidants or specific vitamins, may support overall vascular health, though they should be used under the guidance of a healthcare professional. Some nutraceuticals were also found to help with regenerating glycocalyx. These include Endocalyx (glucosamine) and Arterosil (rhamnan sulfate)
- Avoidance of Toxins: Reducing exposure to harmful toxins, including excessive alcohol, smoking, and environmental pollutants, helps lower the body's overall oxidative stress.
- Proper Sleep: Quality sleep is essential for cellular repair and health, including that of the glycocalyx.
- Control of Blood Sugar Levels: Especially relevant for individuals with diabetes, but also important for general health, maintaining stable blood sugar levels can prevent damage to the glycocalyx associated with hyperglycemia.
In implementing these strategies, an individualized approach is crucial. What works best for one person might not be as effective for another, considering factors like genetics, environment, and personal health history. Consulting with a healthcare professional knowledgeable in holistic health may offer additional personalized strategies for maintaining or improving glycocalyx health.
The bottom line
Though minute and often overlooked, the glycocalyx has an immense impact on kidney health. From maintaining the delicate balance of the GFB to its involvement in the progression of kidney diseases, the importance of this sugar-coated veil cannot be overstated. As we deepen our understanding of glycocalyx and its role in kidney health, we open the door to innovative treatments and better disease management strategies.
Intestinal Dialysis: An Emerging Frontier in Detoxification
The human body is a marvel of biological engineering, with each organ playing its role in maintaining balance and health. When the kidneys falter, we turn to dialysis machines to aid in removing toxins. However, an emerging concept offers an alternative: intestinal dialysis. This method proposes detoxifying the body by using the intestines and their microbial inhabitants. Let's delve into this groundbreaking approach.
By Majd Isreb, MD, FACP, FASN, IFMCP
Intestinal Dialysis and the Gut Microbiota
The gut microbiota, comprising trillions of microbes, has been under extensive research for its role in various physiological functions. Certain bacterial strains have been identified to influence the production or removal of uremic toxins. By modulating the gut microbiota through interventions like probiotics and prebiotics, we can enhance the body's natural detoxification processes.
Oral Adsorbents in Intestinal Dialysis
One of intestinal dialysis's main pillars is using oral adsorbents. These compounds, when consumed, bind to uremic toxins within the intestines, effectively trapping them and facilitating their excretion. Activated charcoal is a well-known adsorbent, but there's also AST-120, a spherical carbon adsorbent. Preliminary studies on AST-120 suggest its potential to delay the progression of chronic kidney disease by adsorbing uremic toxins. However, the full extent of its benefits and safety profile is still under review.
Enhancing Intestinal Clearance
An interesting facet of intestinal dialysis is the potential to increase the removal of uremic solutes directly from the blood into the intestines. This method would entail manipulating certain transporters in the intestines or deploying pharmaceutical agents. The promise here is twofold: not only could this method help in removing toxins, but it might also reduce the burden on failing kidneys.
Direct Intestinal Dialysis Devices
Drawing inspiration from peritoneal dialysis, researchers are looking at the possibility of using the mucosa of the intestines as a semi-permeable membrane for detoxification. Devices would be placed directly within the intestines, functioning similarly to how dialysis machines operate. Although still in nascent stages, this approach could redefine how we view and utilize our gastrointestinal system.
Join us to end the kidney disease epidemic
Challenges and Considerations
While the promise of intestinal dialysis is exciting, it's essential to approach it with a balanced view. Introducing external agents or devices into the gut ecosystem may have unintended consequences. The gut's primary role is digestion and nutrient absorption, and there's a delicate balance of microbial interactions that maintain gut health. Introducing changes to this environment might lead to unforeseen complications, especially for prolonged periods.
The bottom line
Intestinal dialysis is a testament to the ever-evolving field of medical science. New therapeutic avenues are opening up as we continue to unlock the mysteries of the human gut and its microbial residents. Whether it's through direct intestinal devices, oral adsorbents, or microbiota modulation, the dream is to offer patients with kidney diseases a more natural, less invasive detoxification method. With continued research and careful consideration of the challenges ahead, intestinal dialysis might become a mainstay in managing kidney ailments.
October Research and News 2023
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the October Research and News.
Adding urinary biomarkers may improve the prediction of CKD progression
Published in July 2023 edition of The Lancet, this study evaluated the potential of urinary biomarkers in predicting the progression of chronic kidney disease (CKD).
Researchers rigorously tested 30 commercial ELISA assays, focusing on their ability to detect target analytes in urine based on FDA-approved validation criteria.
From these, 16 assays met the approval standards. Using LASSO logistic regression analysis on a subsample of 229 CKD patients, a combination of five specific biomarkers (CCL2, EGF, KIM1, NGAL, and TGF-α) was found to enhance the prediction of a rapid decline in mGFR, offering a superior prediction compared to just using traditional variables such as age, gender, mGFR, and albuminuria.
Why is this important?
Accurate prediction of CKD progression is pivotal for timely medical interventions. This research underscores the enhanced accuracy achieved by combining multiple urinary biomarkers.
Such a comprehensive approach could lead to better therapeutic strategies, improved patient outcomes, and potential cost savings in CKD treatments.
Join us to end the kidney disease epidemic
Inflammatory bowel disease is associated with an increased risk of kidney disease
A study published in August of 2023 sought to determine if there was a connection between inflammatory bowel disease (IBD) and an increased risk of chronic kidney disease (CKD) and acute kidney injury (AKI) within the general population.
For this investigation, data from the UK Biobank, encompassing 417,302 participants (spanning 2006-2010), was analyzed. This included individuals with ulcerative colitis and Crohn's disease but excluded those with prior CKD and AKI.
Over a median duration of 12.5 years, results revealed that participants with IBD had elevated hazard ratios for both CKD and AKI, even after adjusting for a variety of factors, including age, sex, race, biological factors, behavioral tendencies, socioeconomic status, mental health, and self-rated health.
Notably, this association was evident regardless of genetic risks for kidney diseases. Moreover, younger IBD patients exhibited a stronger association with the risks of developing CKD and AKI than their older counterparts.
Why is this important?
This study highlights the role of gut health in the development of kidney disease. This is what we describe as the gut-kidney connection.
Identifying the links between IBD and the risks of CKD and AKI is vital for patient care. Recognizing this association enables healthcare providers to monitor and intervene earlier, potentially preventing or mitigating kidney-related complications in IBD patients. This can significantly improve the prognosis and quality of life for these individuals.
Calprotectin may contribute to vascular calcification in CKD
Calprotectin is a protein found in the white blood cells, primarily neutrophils. This protein is released during inflammation and serves as a marker for several conditions, like inflammatory bowel disease.
Recently, the role of calprotectin in the context of vascular health, especially in patients with chronic kidney disease (CKD), has garnered significant attention.
Chronic kidney disease (CKD) has been linked to enhanced vascular calcification, a factor known to raise the risk of cardiovascular mortality in these patients. However, the precise mechanisms behind this association remained elusive.
In a detailed study led by Amaya-Garrido and her team, they embarked on a proteomic analysis of two sets of patients: those with moderate CKD (stage 3-4) and severe CKD patients on dialysis (stage 5). Their findings spotlighted increased levels of calprotectin as being correlated with a heightened risk of cardiovascular complications and mortality.
They further explored the effects of calprotectin on vascular smooth muscle cells (VSMCs), wherein its presence exacerbated calcification.
Interestingly, when treated with a calprotectin inhibitor called paquinimod, this calcification could be staved off. This inhibitory effect extended to mice with CKD induced by nephrectomy and even aged mice deficient in apolipoprotein E.
This extensive research underscores the pivotal role of calprotectin in driving vascular calcification, with its levels in the bloodstream being strongly linked to calcification severity, cardiovascular outcomes, and mortality in CKD patients.
Why is this important?
The discovery of calprotectin's role in vascular calcification in CKD patients presents a new avenue for potential therapeutic interventions.
With vascular calcification being a significant contributor to cardiovascular mortality in CKD patients, strategies inhibiting calprotectin may offer a promising approach to curb this risk, improving the overall prognosis for these individuals.
Simple, low-cost intervention proves effective in reducing salt intake in CKD patients
Managing dietary habits, specifically salt intake, is crucial for people diagnosed with CKD. High salt intake can exacerbate blood pressure issues and potentially accelerate kidney damage in these patients.
In a recent multicenter randomized controlled trial, O'Callaghan and his team delved into the effectiveness of a simple, inexpensive intervention to empower CKD patients to reduce their salt intake. This initiative termed the OxSalt care bundle intervention, was tested against standard care over a month.
The study participants were CKD patients with an eGFR greater than 20 ml/min per 1.73 m^2, enlisted from primary and secondary care facilities.
The primary objective was to measure the reduction in salt consumption, gauged by a 24-hour urinary sodium excretion test after a month of the intervention. Out of the 201 participants, those in the intervention group witnessed a notable drop in their salt intake by 1.9 (±2.9) g/d, a stark contrast to the 0.4 (±2.7) g/d decline in the control group.
Interestingly, the intervention group still demonstrated a decrease, though marginal, in their salt intake in the subsequent year.
Why is this important?
Dietary salt reduction plays a role in the management and progression of CKD. The findings from this study underline the potential of a basic, cost-effective intervention in instigating a positive dietary change among CKD patients.
Such scalable and easy-to-implement interventions can have far-reaching benefits in improving the health and well-being of CKD patients across diverse care settings.
The persistent effect observed even after the intervention ended adds further weight to the potential long-term impact of the OxSalt initiative. You can download the OxSalt intervention here.
Review Article of the Month
Subtle changes in platelet count and volume may indicate risk of bleeding or clotting in CKD
A perspective article in JASN noted that subtle changes in platelet count and platelet volume may indicate which patients with CKD are at risk of bleeding or thrombosis.
It recommends complete blood counts for predicting such risk to optimize the use of anti-platelet drugs in this patient population.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The adrenal-kidney connection
The human body functions as an intricate network, where each component plays its pivotal role. At the heart of this network lie the adrenal glands and the kidneys. This article delves into the 'adrenal-kidney connection,' emphasizing the interplay between these vital organs and their overarching importance to our health.
Whether you're a medical professional, someone eager to gain a deeper understanding of your own body, or searching for insights into health-related queries, this piece will shed light on the essential relationship between the adrenal glands and the kidneys. Join us as we explore their roles, synergies, and their collective influence on our well-being.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Adrenal Glands: Hormonal Powerhouses
Tucked above each of our kidneys are the adrenal glands, small yet incredibly powerful organs responsible for producing a variety of hormones essential for our body's function.
Mineralocorticoids:
- Primary hormone: Aldosterone.
- Function: Aldosterone plays a pivotal role in regulating the balance of sodium and potassium in our blood. This balance is essential for controlling blood volume, blood pressure, and the pH balance of our body fluids.
Glucocorticoids:
- Primary hormone: Cortisol.
- Function: Often dubbed the 'stress hormone,' cortisol helps our body respond to stress. It also plays a role in regulating metabolism, reducing inflammation, and controlling our sleep-wake cycle. Our body's glucose levels are also managed by cortisol, as it promotes the production of glucose from proteins in our liver.
Sex Hormones:
- Adrenal Androgens: DHEA (Dehydroepiandrosterone) and Androstenedione.
- Function: Although the adrenal glands produce only a small quantity of male and female sex hormones (with the testes and ovaries being the primary producers), these hormones are essential, especially before puberty. They assist in the development of secondary sexual characteristics like pubic hair. Post-menopause, the adrenal glands become the primary source of estrogen in women.
Cortisol: The Multifaceted Stress Hormone
Cortisol, the primary glucocorticoid hormone churned out by the adrenal glands, is widely recognized as the "stress hormone." While its release intensifies during stressful periods, its daily functions go beyond just responding to stress and are vital for overall human health.
- Glucose production: Cortisol boosts glucose generation from non-carbohydrate sources. This ensures a consistent energy flow, particularly during high-stress moments.
- Anti-inflammatory actions: Cortisol acts as a brake on inflammation. It achieves this by inhibiting the release of pro-inflammatory agents in the body.
- Blood pressure regulation: Cortisol indirectly hikes up blood pressure by making blood vessels more receptive to hormones that cause constriction.
- Water balance: In tandem with aldosterone, cortisol orchestrates the body's water distribution and balance.
- Circadian rhythm: The levels of cortisol ebb and flow, peaking just before we wake up in the early hours and dipping to their nadir at night. This rhythm profoundly impacts our sleep-wake cycle.
A testament to cortisol's widespread influence is the presence of glucocorticoid receptors (GRs) in nearly every cell of our body. Once cortisol binds to these receptors, the resulting complex can move into the cell nucleus and influence gene transcription, leading to various cellular responses.
Effects of Excess Cortisol
Chronic overproduction of cortisol, or hypercortisolism, can lead to a condition called Cushing's syndrome. Some potential effects and symptoms include:
- Weight Gain: Particularly around the abdomen and face.
- Muscle Weakness: Due to the catabolic effects of cortisol on muscle tissue.
- Osteoporosis: Increased bone resorption can reduce bone density.
- Hypertension: Elevated cortisol levels can chronically elevate blood pressure.
- Impaired Immune Function: Reduced capability of the body to fight off infections.
- Mood Disorders: Anxiety, depression, and irritability can become more prevalent.
- Hyperglycemia: High blood sugar and potential development of diabetes due to increased glucose production.
- Skin Changes: Thinning skin, easy bruising, and purple stretch marks.
- Cardiovascular disease and death: Even a mild degree of persistent elevated cortisol exposure profoundly impacts cardiovascular disease and mortality.
The HPA Axis: Understanding the dynamics of cortisol function
The Hypothalamic-Pituitary-Adrenal (HPA) axis constitutes a sophisticated interplay between three principal glands: the hypothalamus, the pituitary gland, and the adrenal glands. This system plays a critical role in controlling reactions to stress, digestion, the immune system, mood and emotions, sexuality, and energy storage.
Here's a brief walkthrough of the process:
- Stress Perception: Upon detecting stress or other stimuli, the hypothalamus secretes corticotropin-releasing hormone (CRH).
- Pituitary Activation: CRH then galvanizes the pituitary gland to release adrenocorticotropic hormone (ACTH).
- Adrenal Response: ACTH interacts with the adrenal cortex, spurring it to synthesize and release cortisol. Notably, cortisol also serves as a regulatory checkpoint: once released, it suppresses the production of both CRH and ACTH, establishing a feedback loop that prevents excessive cortisol release.
The secretion of cortisol isn’t arbitrary; our circadian rhythm finely tunes it. Cortisol levels peak in the early morning hours (around 8 a.m.) and drop to their lowest at midnight. This rhythm ensures our body is alert, ready for the day's challenges, and winds down as nighttime approaches.
About 90% of cortisol in the blood is bound to a protein called corticosteroid-binding globulin (CBG), with the remaining 10% being free or unbound. Only the unbound cortisol is biologically active.
Cortisol has a relatively short half-life of approximately 60-90 minutes. The liver metabolizes cortisol, converting it into inactive metabolites, including cortisone. These metabolites are then excreted in the urine via the kidneys.
To strike a balance between cortisol and cortisone in the body, two pivotal enzymes come into play:
- 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1): Converts cortisone into the active cortisol. This enzyme utilizes a cofactor called NADPH (nicotinamide adenine dinucleotide phosphate) and is mainly present in metabolically active tissues such as the liver, fat, and skeletal muscles.
- 11β-Hydroxysteroid Dehydrogenase Type 2 (11β-HSD2): Converts cortisol back into the inactive cortisone. The cofactor for this enzyme is NAD+ (nicotinamide adenine dinucleotide). It is highly expressed in the kidneys. This is particularly important in mineralocorticoid target tissues, ensuring the prevention of excessive activation of the mineralocorticoid receptors by cortisol.
Finally, while the amount of circulating cortisol is 10 times that of cortisone, cortisol has a higher affinity to bind to CBG. Therefore, the amount of free cortisol in the blood is comparable to that of free cortisone.
Join us to end the kidney disease epidemic
The adrenal kidney-connection
Cortisol levels in CKD
Chronic Kidney Disease (CKD) has a complex interplay with cortisol. Researchers have delved into how CKD affects cortisol levels, yielding the following insights:
- Early morning cortisol levels: Studies measuring early morning blood cortisol in CKD patients have shown mixed results.
- Cortisol circadian rhythm: A consistent finding in medical research has been the presence of elevated evening cortisol levels, especially in stage 3 CKD and beyond.
- Saliva Cortisol Levels: This test, indicative of serum-free active cortisol, echoes the finding of high evening cortisol levels in CKD.
- 24-hour urine collection: This method measures the total cortisol and its metabolites in the urine. Intriguingly, it shows that the daily cortisol output from the adrenal glands remains relatively unchanged in mild to moderate CKD. This phenomenon might stem from CKD's reduced clearance of free cortisol, rendering the 24-hour urine free cortisol measurement unreliable for detecting cortisol excess in CKD patients.
- Protein binding and cortisol: Data suggests that cortisol's protein binding to CBG remains stable in CKD.
- Changes in the HPA axis feedback: While the adrenal glands' response to ACTH remains intact in CKD, cortisol levels display a partial resistance to suppression by dexamethasone - a compound typically used to probe the HPA axis's negative feedback mechanism. This resistance can be overcome with higher dexamethasone doses or longer exposure, pointing to a diminished negative feedback mechanism in CKD, essential for modulating adrenal cortisol output.
- Prolonged cortisol half-life in CKD: The half-life of circulating cortisol is extended in CKD for various reasons, such as the buildup of cortisol metabolites due to hampered renal function, reduced cortisol breakdown in the liver, and diminished activity of the enzyme 11β-HSD2, attributed to loss of kidney tissue.
Effects of elevated cortisol levels in CKD
Cortisol plays a complex role in the setting of CKD. Elevated cortisol levels bring forth a range of effects:
- Mortality risks: Multiple studies spotlight the association of raised cortisol levels with an uptick in all-cause and cardiovascular mortality. Even slight elevations in cortisol can be linked to such adverse outcomes.
- Changes in kidney function:
- Short-Term Effects: Administering ACTH or cortisol in the short term has been seen to increase glomerular filtration rates (GFR) in humans. Conversely, diminished cortisol levels correlate with a drop in kidney blood flow and GFR.
- Long-Term Impacts: On the other hand, chronic exposure to high cortisol concentrations can lead to a decline in kidney functionality. Alarmingly, individuals with Cushing’s disease (characterized by cortisol excess) exhibited compromised kidney function, a condition persisting even post-treatment. The disease's duration has been pinpointed as a key factor affecting kidney health.
- Metabolic changes: High cortisol levels can incite insulin resistance and alterations in blood lipid profiles. Such shifts enhance susceptibility to diabetes and metabolic syndrome. These two factors can further worsen kidney function.
- Other consequences in CKD: Elevated cortisol levels in CKD patients manifest in various other ways:
-
- Muscle Mass: A discernible loss of muscle mass.
- Nutrition: Deteriorated nutritional status.
- Immune System: A compromised immune function.
- Mental Health: Increased likelihood of depression.
- Sleep Patterns: Disrupted and poor-quality sleep.
Strategies to Lower Cortisol Levels in CKD
Ensuring a balance in stress and hormones is pivotal for overall health, especially in individuals with CKD. Here are several natural strategies to mitigate elevated cortisol levels, particularly in the evenings:
- Relaxation techniques: Incorporating consistent relaxation practices, such as deep breathing exercises, meditation, and gentle yoga, can substantially curtail stress-driven spikes in cortisol.
- Sleep hygiene:
- Consistency: Stick to a regular sleep schedule.
- Environment: Prepare for sleep in a dimly lit, calm setting.
- Tech Break: Avoid electronic screens at least an hour before bedtime. This practice aids in realigning the body's circadian rhythm, potentially tempering evening cortisol surges.
- Dietary adjustments:
- Nutrient-rich foods: Prioritize a diet abundant in anti-inflammatory ingredients and omega-3 fatty acids. Inflammation, which can be controlled through diet, is a significant catalyst for elevated cortisol in CKD.
- Caffeine Caution: Limit caffeine consumption, especially during the latter part of the day.
- Supplements: supplements such as fish oil and Ashwagandha have been found to decrease cortisol levels.
- Light therapy: An often-underestimated tool, light therapy can be transformative in regulating cortisol levels. Light exposure deeply influences our circadian rhythm (10,000 lux; equivalent to ambient light intensity just after sunrise). Utilizing morning light therapy devices, which emulate natural sunlight, can recalibrate this internal clock. As a result, cortisol levels witness a steadier, more organic drop come evening.
- Addressing metabolic acidosis: In CKD, metabolic acidosis and an increased dietary acid load have been linked to elevated cortisol levels. Rectifying this by embracing an alkaline diet and curtailing animal protein consumption can be beneficial in moderating cortisol levels.
- Finally, moderate exercise, healthy relationships, laughing, and taking care of pets can also relieve stress and decrease cortisol levels.
The Bottom line
The intricate relationship between the adrenal glands and the kidneys plays a critical role in our overall health, with cortisol acting as a central figure in this dance. Elevated cortisol levels, especially in the context of CKD, have multifaceted implications, from metabolic changes to compromised kidney function. Addressing these elevated levels is vital, and methods such as stress management, dietary adjustments, light therapy, and improved sleep hygiene offer promising avenues. As research continues to delve deeper into this adrenal-kidney connection, it becomes increasingly evident that a comprehensive, holistic approach to managing cortisol is paramount for the well-being of CKD patients and everyone alike.
The Connection Between Dementia and Chronic Kidney Disease
Dementia and Chronic Kidney Disease (CKD) are two conditions that have attracted significant attention in the medical community. Though they might seem unrelated at first glance, recent studies have begun to uncover links between the two. This connection is critical to understanding the full scope of these conditions and how they might impact one another.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Prevalence of Dementia in CKD
Kidney failure can lead to cognitive disturbances, affecting mental clarity in individuals. Those with chronic kidney disease may experience varying degrees of cognitive impairment, from mild disruptions to severe debilitations. When this impairment profoundly disrupts daily activities, it leads to dementia.
Early studies found that 13% of patients with CKD have cognitive impairment. The prevalence increases with the advancing stages of CKD independent of the traditional vascular risk factors. However, recent studies found that cognitive impairment affects as many as 62.5% of individuals with chronic kidney disease.
Understanding the Link
Researchers have begun to explore the connection between dementia and CKD, and several theories have emerged.
Vascular Factors
Vascular factors are common in both dementia and CKD, with blood vessel damage playing a significant role. The restriction of blood flow may lead to cognitive decline, and kidney function might also be compromised. In addition, CKD is often associated with vascular dysfunction, arterial stiffness and calcifications, enhanced systemic inflammation, and oxidative stress. All of these can lead to cognitive deficits.
Join us to end the kidney disease epidemic
Uremic Toxins
CKD can lead to the buildup of uremic toxins in the body, affecting various systems, including the brain. These toxins contribute to cognitive impairment and dementia. Uremic toxins, such as indoxyl sulfate, may play a role in cognitive function decline through direct toxicity or indirectly through vascular calcification, endothelial dysfunction, oxidative stress, and inflammation. Some uremic toxins can disrupt the blood-brain barrier, causing further cognitive decline.
Shared Risk Factors
Both conditions share common risk factors, such as hypertension, insulin resistance, and obesity. These conditions may simultaneously increase the risk of developing dementia and CKD, linking them to a complex web of cause and effect.
Management and Prevention
Understanding the connection between dementia and CKD helps in devising strategies to manage and prevent these conditions.
Early Detection
Screening for CKD in individuals with dementia or cognitive impairment can aid early detection and intervention, possibly slowing the progression of both diseases.
Holistic Treatment
Adopting a holistic approach to treating both conditions can ensure they are managed simultaneously, recognizing that they may influence each other.
Lifestyle Modifications
Healthy living, including a balanced diet, regular exercise, and avoiding tobacco, can reduce the risk of developing both dementia and CKD or at least slow their progression. The strategies often overlap, emphasizing a holistic approach to overall health and wellness.
- Diet: A nutritious diet, rich in fiber, antioxidants, omega-3 fatty acids, and low in saturated fats, can boost cognitive health. The Mediterranean diet, for example, has been associated with a reduced risk of dementia. This diet was also recently found to slow the progression of CKD. In CKD, the diet has additional attention to sodium, phosphate, and oxalate content. Monitoring protein intake is also essential.
- Physical Activity: Regular exercise increases blood flow to the brain and promotes cognitive function. It can include walking, jogging, swimming, or other aerobic activities. These can also improve BP control and insulin resistance. Regular exercise was found to slow the progression of kidney disease.
- Stress Management: Chronic stress may increase dementia risk. It was also found to increase the risk for CKD. Meditation, deep breathing exercises, or hobbies can help in managing stress.
- Avoidance of Toxins: Limiting alcohol and avoiding smoking helps to protect cognitive and kidney health. In addition, avoiding over-the-counter medications such as NSAIDs and environmental toxin exposure can help both.
- Blood Pressure Control: Managing blood pressure through diet, exercise, and medication, if needed, is crucial for kidney health. It is also important for vascular and cognitive health.
- Insulin resistance: Many of the above factors can help improve insulin resistance. However, in addition to that, decreasing carb intake, improving sleep, and losing weight can be essential. Reversing insulin resistance can improve cognitive and kidney health.
- Mental Stimulation: Engaging in intellectually stimulating activities like puzzles, reading, or learning a new skill helps to keep the mind sharp. This may be unique to dementia.
The Bottom Line
The connection between dementia and CKD is a complex and multifaceted issue that requires more research to fully understand. However, the links that have been discovered thus far provide crucial insights that can help manage and prevent these conditions. By recognizing that these seemingly disparate conditions are interconnected, healthcare providers can take a more integrative and effective approach to treatment, ultimately benefiting those who suffer from these debilitating diseases.
The Woven Threads of Klotho: Unraveling the Role of Klotho in Kidney Health and Vascular Calcifications
In Greek mythology, Clotho, one of the three Fates, spun the threads of human life, intricately weaving destiny and lifespan into every fiber. Inspired by this symbolism, scientists named an essential human protein "Klotho." This protein plays a vital role in regulating aging, kidney health, and cardiovascular functions. Klotho, predominantly produced by the kidneys, interplays with multiple physiological processes, offering a fascinating field of study and potential therapeutic intervention in chronic kidney disease (CKD) and vascular calcifications. In this blog, we will discuss the role of Klotho in kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is Klotho?
Klotho is a group of proteins that span across cell membranes. They function as “co-receptors” to various fibroblast growth factors (FGFs). Since the discovery of the Klotho gene in 1997, there have been many breakthroughs in the understanding of these proteins.
There are also soluble forms of Klotho that can be present in the systemic circulation. These soluble forms can act as endocrine “hormones” with many functions. They have been found to have antioxidant and anti-inflammatory properties. In addition, they have been found to prevent tissue fibrosis.
Deficiency in alpha-Klotho, which we will describe from here on as Klotho, has been linked with features of aging, kidney disease, vascular calcifications, and cardiovascular disease.
The role of Klotho in kidney health
A deeper understanding of Klotho's function begins with its role in the kidneys, where it helps maintain mineral balance. Phosphate plays a vital role in many physiological processes. Maintaining its balance is essential for bone health, energy production, and cellular functions. At the center of phosphate regulation is the Klotho.
Klotho functions as a co-receptor for fibroblast growth factor 23 (FGF23), a bone-derived hormone. This partnership between Klotho and FGF23 is intrinsic to the fine-tuning of phosphate balance.
When phosphate levels are elevated, FGF23 levels rise, prompting the kidneys to excrete more phosphate. For FGF23 to act effectively on the kidneys, the presence of Klotho is indispensable. They synergize to activate a signaling pathway that regulates the reabsorption of phosphate in the kidneys. Without adequate Klotho, this mechanism becomes inefficient, resulting in phosphate retention and potentially leading to hyperphosphatemia (elevated serum phosphate levels).
In the context of CKD, Klotho expression often decreases, leading to increased serum phosphate levels. This imbalance can contribute to vascular calcification and other complications associated with CKD.
Klotho and vascular calcifications
Vascular calcification is a common complication in CKD and is a significant predictor of cardiovascular morbidity and mortality. Research has linked Klotho deficiency to vascular calcification, particularly in the CKD context. Increased phosphate levels can drive calcium buildup in blood vessel walls, a condition exacerbated by the kidneys' reduced ability to produce Klotho in CKD patients.
Klotho can serve as a protective agent. By inhibiting phosphate uptake by vascular smooth muscle cells and promoting phosphate excretion via its interaction with FGF23, Klotho mitigates the triggers of vascular calcification. This important function, seen in animal models, presents a promising therapeutic target for managing CKD-associated vascular calcification.
The antiaging properties of Klotho
The benefits of Klotho go beyond kidney and vascular health. There have been many research studies that found that it has tumor-suppressive properties, anti-inflammatory activities, antioxidant benefits, and anti-diabetic actions. In addition, Klotho has been associated with cognitive deficit disorders such as Alzheimer’s disease.
Factors affecting Klotho expression
Various factors can influence Klotho's expression, both positively and negatively. Aging, inflammation, oxidative stress, and certain medications have been associated with decreased Klotho expression. Conversely, some interventions, such as exercise and caloric restriction, can potentially increase Klotho expression.
Vitamin D can also increase the expression of Klotho. In addition, a natural chemical compound called Ligustilide has been found to increase circulating soluble Klotho. Understanding these dynamics may offer ways to manipulate Klotho levels for therapeutic benefit, especially in CKD. Interestingly, even FGF23 itself can lower Klotho’s expression.
Many medications have also been found to increase Klotho.
Join us to end the kidney disease epidemic
Optimizing Klotho for better kidney health
Many factors can help optimize the levels of Klotho to improve the outcome of kidney disease. These may also be “anti-aging” factors:
- Exercise: Many studies have demonstrated that exercise can increase the level of circulating Klotho. Regardless of the type of exercising, only 3 months have been documented to increase the levels of Klotho.
- Optimizing vitamin D level: Vitamin D supplementation has been found to normalize Klotho levels in children with mild to moderate CKD. Many animal studies have also shown that vitamin D can enhance the expression of Klotho.
- Limiting phosphate intake: Higher phosphate intake is the major stimulus for the production of FGF23 by the bone. Higher FGF23 levels decrease the expression of Klotho. Therefore, limiting phosphate intake from ultra-processed food can improve Klotho levels.
- Diet: Caloric restriction and intermittent fasting have been found to enhance the expression of Klotho. In addition, food that is high in Ligustilide, such as wild celery, can increase Klotho.
- Various supplements were also found to increase Klotho. These include resveratrol, ginseng, astaxanthin, baicalin, cordyceps, and curcumin.
The myth of Clotho: Spinning the thread of life
Just as the Greek Fate Clotho spun the thread of life, determining each individual's destiny and lifespan, Klotho, in its physiological role, impacts our health and longevity. The similarity goes beyond their shared responsibility for life's thread; neither can be fully controlled. Clotho's threads were immutable, not even subject to the will of the gods. Similarly, the science of Klotho is complex, and its manipulation challenging, though potentially rewarding.
The bottom line
Much like Clotho's threads, the intricate roles of Klotho continue to be unraveled by researchers. From regulating kidney health to potential implications in mitigating vascular calcifications, Klotho holds a significant place in our physiological understanding. As our knowledge deepens, so too does the promise of leveraging this protein for therapeutic interventions, offering a potential lifeline to those battling chronic kidney disease and associated complications.