October Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the October Research and News.
Cannabis Cream Reduces Itching in CKD Patients: A Randomized Controlled Trial
This double-blind, placebo-controlled study evaluated the effectiveness of a cannabis-containing cream in alleviating pruritus associated with chronic kidney disease (CKD).
Sixty hemodialysis patients experiencing moderate to severe itching were randomly assigned to use either the cannabis cream or a placebo over four weeks.
The primary measure was the change in itching intensity, assessed using the Worst Itching Intensity Numerical Rating Scale (WI-NRS).
Results showed a significant reduction in itch severity in the cannabis group compared to the placebo, with no reported side effects.
Why is this important?
Itching is a common and distressing symptom for patients with CKD, often leading to reduced quality of life. Current treatments are limited in effectiveness and can have undesirable side effects.
The findings suggest that cannabis cream could be a safe and effective option for managing CKD-associated pruritus, providing relief and potentially improving overall well-being in this patient population.
Further research with larger sample sizes and longer follow-up periods could help validate these results and explore the long-term safety and effectiveness of cannabis-based treatments for pruritus in CKD patients.
"Weekend Warrior" Physical Activity Reduces Kidney Disease Risk: A Study of 77,977 Participants
A comprehensive analysis of 77,977 UK Biobank participants explored the impact of a "weekend warrior" physical activity pattern—where most moderate to vigorous physical activity (MVPA) is packed into 1-2 days a week—on the incidence of chronic kidney disease (CKD) and acute kidney injury (AKI).
Participants were categorized into three groups: active weekend warriors, active regulars (evenly distributed exercise), and inactives (less than 150 minutes of MVPA per week).
The findings indicated that both active weekend warriors and regular exercisers had a lower risk of developing CKD and AKI compared to inactive participants, demonstrating that concentrated weekend activity is as beneficial as more frequent exercise sessions.
Why is this important?
This study challenges the traditional notion that exercise must be distributed evenly throughout the week to confer health benefits, particularly concerning kidney health.
The "weekend warrior" exercise pattern, often more feasible for individuals with busy weekday schedules, is now shown to be equally effective in reducing the risks of CKD and AKI.
This flexibility in achieving physical activity goals can make lifestyle interventions more accessible and manageable for the wider public, potentially improving adherence rates and public health outcomes related to kidney disease.
Increased Risk of Falls, Hospitalizations, and Mortality Linked to Discrepancy in Kidney Function Estimates Among Older Adults
A comprehensive study involving 5,574 older adults from the Health and Retirement Study investigated the health risks associated with significant discrepancies between creatinine-based and cystatin C-based estimated glomerular filtration rates (eGFR).
Defined as a cystatin-based eGFR (eGFRcys) being more than 30% lower than the creatinine-based eGFR (eGFRcr), a large discordance was observed in 30% of the participants.
Over two years, those with a large eGFR discordance faced increased risks of death, falls, and hospitalizations, though not hip fractures.
Why is this important?
When cystatin C-based eGFR is more than 30% lower than creatinine-based eGFR in elderly individuals, this discrepancy could indicate reduced muscle mass.
Since creatinine production is influenced by muscle mass, less muscle results in lower creatinine levels, potentially leading to an overestimation of renal function when using creatinine-based eGFR alone.
This makes cystatin C—a biomarker not affected by muscle mass—a valuable tool for more accurately assessing kidney function in this demographic.
This understanding is crucial as it highlights the need for dual filtration markers to improve diagnostic accuracy and guide better clinical decisions in managing elderly patients, potentially leading to more appropriate and targeted interventions.
Join us to end the kidney disease epidemic
Urinary Response to Consuming Plant-Based Meat Alternatives in Persons with Normal Kidney Function: The SWAP-MEAT Pilot Trial
The SWAP-MEAT trial, a randomized eight-week crossover study, evaluated the impact of plant-based meat alternatives compared to traditional animal meat on urinary and serum markers in individuals with normal kidney function.
The trial included 36 participants who alternated between consuming "plant-meat" and animal meat over two eight-week phases.
Results showed that plant-meat consumption led to significantly lower levels of urinary sulfate, ammonium, phosphorus, and urea nitrogen, alongside higher urine pH and citrate/creatinine ratios.
Additionally, serum creatinine concentrations decreased slightly during the plant-meat phase, although serum bicarbonate levels remained unchanged.
Why is this important?
Switching from animal to plant-based meat alternatives could potentially lower the dietary acid load and modify urinary excretion profiles in ways that may benefit kidney health.
These findings suggest that plant-based diets might help in managing or preventing conditions exacerbated by higher dietary acid loads, such as urinary stone disease and chronic kidney disease.
Further research could explore whether these dietary changes would have practical therapeutic implications for individuals with compromised kidney function, particularly in light of the global rise in kidney disease prevalence and the need for preventive dietary strategies.
Review article of the month
Toxic Exposures in Vulnerable Populations
The health of our environment significantly impacts community and individual health. Exposure to both man-made and natural toxins—like air, water, and soil pollutants—can cause tissue damage, organ dysfunction, and increase morbidity and mortality rates.
Common pollutants include fine particulate matter, heavy metals such as arsenic and mercury, pesticides, herbicides, and perfluorinated chemicals. Furthermore, increasing global incidences of heat waves, wildfires, and other natural disasters not only directly affect health but also lead to higher exposure to mixed pollutants and infrastructure damage.
This editorial discusses all these details.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Integrative Approach to Diabetes and Insulin Resistance: A Holistic Path to Better Health
Diabetes and insulin resistance have become pressing global health issues, affecting millions of people worldwide. Diabetes is the leading cause of chronic kidney disease. While conventional medicine focuses primarily on managing blood sugar levels through medication and lifestyle modifications, the integrative approach to diabetes and insulin resistance emphasizes a more holistic strategy. By combining conventional treatment with complementary therapies, addressing the underlying causes, and promoting overall well-being, this approach aims to not only manage symptoms but also improve long-term outcomes. This post will explore how an integrative approach can provide more comprehensive care for individuals with diabetes and insulin resistance.
As usual, the references are in the hyperlinks.
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding Insulin Resistance and Diabetes
Before exploring the integrative approach, it's essential to understand the relationship between insulin resistance and diabetes. Insulin resistance occurs when cells in the muscles, fat, and liver stop responding properly to insulin, a hormone that helps regulate blood sugar. This resistance causes the pancreas to produce more insulin to compensate, leading to higher blood sugar levels. Over time, insulin resistance can lead to prediabetes and type 2 diabetes.
In conventional medicine, diabetes is often managed with medications such as metformin and insulin, along with dietary changes and exercise. While effective in controlling blood sugar, these strategies don’t always address the root causes or the whole-body impacts of the disease. This is where the integrative approach to diabetes and insulin resistance can offer added benefits.
The Role of an Integrative Approach to Diabetes and Insulin Resistance
The integrative approach is rooted in the belief that diabetes is a multi-faceted condition influenced by a variety of factors, including genetics, environmental toxins, gut health, inflammation, and lifestyle. The goal is to combine evidence-based treatments from conventional medicine with complementary therapies that support the body’s natural healing processes.
Addressing Nutrition and Diet
One of the key pillars of the integrative approach to diabetes and insulin resistance is nutrition. While conventional care recommends cutting back on processed foods and sugars, integrative care goes a step further by tailoring dietary interventions based on the individual’s unique needs. Functional nutrition emphasizes whole, nutrient-dense foods that reduce inflammation, support gut health, and promote balanced blood sugar levels.
For example, diets rich in omega-3 fatty acids, antioxidants, and fiber can be especially beneficial. The Mediterranean diet, which is rich in fruits, vegetables, whole grains, and healthy fats, is commonly recommended for people with insulin resistance. In addition, some people with diabetes may benefit from intermittent fasting or a ketogenic diet, which promotes fat-burning and improved insulin sensitivity.
Join us to end the kidney disease epidemic
Gut Health and Its Connection to Insulin Resistance
Emerging research has shown that gut health plays a critical role in metabolic disorders, including diabetes and insulin resistance. The integrative approach to diabetes and insulin resistance places a strong emphasis on restoring gut health. An imbalanced gut microbiome can contribute to systemic inflammation, which is a key driver of insulin resistance.
A comprehensive gut restoration protocol such as the 5R protocol supports a healthy gut and can help improve metabolic function. Foods such as fermented vegetables, yogurt, and fiber-rich foods are important for fostering a diverse microbiome. In some cases, practitioners may recommend specific probiotic supplements to help restore balance in the gut as a part of a broader protocol.
Stress Management and Mind-Body Techniques
Chronic stress is another major factor that contributes to insulin resistance and diabetes. Stress hormones, such as cortisol, can increase blood sugar levels and exacerbate insulin resistance. The integrative approach to diabetes and insulin resistance incorporates mind-body practices like mindfulness, meditation, yoga, and tai chi to help reduce stress.
These techniques not only improve mental and emotional well-being but can also have a direct impact on blood sugar control. For instance, mindfulness-based interventions have been shown to lower hemoglobin A1c levels, which measure long-term blood sugar control.
Physical Activity and Movement
While conventional treatment plans for diabetes include regular physical activity, the integrative approach goes beyond prescribing exercise as a way to burn calories. It promotes forms of movement that enhance overall wellness, improve insulin sensitivity, and reduce inflammation. Practices such as yoga, Pilates, and tai chi are not only good for the body but also for the mind. These activities improve flexibility, balance, and relaxation while supporting metabolic health.
In addition, strength training and resistance exercises are often recommended in an integrative plan, as they can increase muscle mass and improve insulin sensitivity. Tailored exercise regimens can further enhance the body's ability to regulate blood sugar levels and improve overall fitness.
Environmental Toxins and Diabetes
One unique aspect of the integrative approach to diabetes and insulin resistance is its focus on environmental toxins. Increasing evidence shows that exposure to certain chemicals, such as bisphenol A (BPA), phthalates, and heavy metals, can disrupt metabolic processes and contribute to insulin resistance. Functional medicine practitioners may recommend detoxification strategies, including the use of specific supplements, infrared sauna therapy, and dietary changes to reduce toxic burden.
Reducing exposure to endocrine-disrupting chemicals and supporting the body's natural detoxification pathways can help individuals with insulin resistance manage their condition more effectively.
Integrating Conventional and Complementary Therapies
The integrative approach to diabetes and insulin resistance is not about replacing conventional treatments but rather enhancing them with complementary strategies. For example, someone with diabetes may continue taking prescribed medications while also incorporating acupuncture, herbal medicine, or supplements like berberine, chromium, or alpha-lipoic acid (note alpha lipoid acid may trigger membranous nephropathy), which have been shown to support blood sugar regulation.
Acupuncture, for instance, has been found to improve insulin sensitivity and reduce inflammation. Herbal remedies like cinnamon and fenugreek have been used to regulate blood sugar levels and improve insulin function. It is important to work with a healthcare provider trained in integrative medicine to ensure that these complementary therapies are safe and effective.
Join us to end the kidney disease epidemic
The Bottom Line on the Integrative Approach to Diabetes and Insulin Resistance
A Personalized, Holistic Approach
In summary, the integrative approach to diabetes and insulin resistance is a holistic strategy that addresses the root causes of the condition while promoting overall health and well-being. By combining conventional treatments with functional nutrition, stress management, gut health support, detoxification, and physical activity, this approach offers a comprehensive and personalized plan for managing diabetes and improving insulin sensitivity. It encourages individuals to take an active role in their health and empowers them to make choices that lead to long-lasting improvements in their quality of life.
Assessing Gut Health for Kidney Disease Management: Comprehensive Methods
The connection between gut health and kidney health is an emerging area of research, revealing a significant interplay between the two. The gut-kidney axis, a bidirectional relationship between the gastrointestinal tract and the kidneys, plays a crucial role in the progression and management of kidney disease. Understanding and assessing gut health can provide valuable insights for optimizing kidney disease management. This blog explores various methods for assessing gut health for kidney disease management, including questionnaires, blood tests, stool tests, and urine tests.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Gut-Kidney Axis: An Overview
The gut and kidneys are intricately linked through complex metabolic, immunological, and hormonal pathways. Disruptions in gut health, such as dysbiosis (an imbalance in the gut microbiota), can produce harmful substances like uremic toxins, which negatively impact kidney function. Conversely, kidney disease can alter gut microbiota composition, leading to further complications. Therefore, assessing gut health is essential for a holistic approach to managing kidney disease.
Assessing Gut Health for Kidney Disease Management: Key Methods
Questionnaires and Dietary Assessments
Questionnaires are an accessible and straightforward method for evaluating gut health, especially in relation to diet and lifestyle factors. These tools help identify patterns and behaviors that may contribute to gut health issues and, by extension, kidney health problems.
- Dietary Habits: Questionnaires can assess dietary patterns, focusing on fiber intake, consumption of prebiotics and probiotics, and the presence of processed foods, which can influence gut microbiota. A diet high in fiber and low in processed foods supports a healthy gut microbiome, which benefits kidney health.
- Gastrointestinal Symptoms: Patients with kidney disease often experience gastrointestinal symptoms such as bloating, constipation, and diarrhea. Questionnaires that capture these symptoms can provide insights into underlying gut health issues that may need to be addressed as part of kidney disease management.
- Lifestyle Factors: Factors such as stress, sleep patterns, and physical activity also influence gut health. Questionnaires can evaluate these aspects, helping to create a comprehensive picture of the patient's gut health and its potential impact on kidney function.
Our free questionnaire can be used to cover all these aspects.
Blood Tests for Inflammatory Markers
Blood tests can assess gut health for kidney disease management by measuring inflammatory markers and other substances that reflect the state of the gut-kidney axis. Inflammation is a common thread connecting gut dysbiosis and kidney disease progression.
- C-Reactive Protein (CRP): High-resolution CRP (hs-CRP) is an inflammatory marker that can be elevated in gut dysbiosis and kidney disease. High levels of CRP may indicate systemic inflammation linked to poor gut health, which could exacerbate kidney damage.
- Interleukin-6 (IL-6): IL-6 is another inflammatory marker associated with gut-derived inflammation. Elevated IL-6 levels in the blood can suggest the presence of gut dysbiosis, which may negatively affect kidney health.
- Endotoxins: Blood tests can also measure endotoxins, such as lipopolysaccharides (LPS), which are released by certain gut bacteria. Elevated levels of LPS in the bloodstream indicate a compromised gut barrier, often referred to as intestinal hyperpermeability "or leaky gut," which can lead to systemic inflammation and worsen kidney disease.
Stool Tests for Gut Microbiota Analysis
Stool tests are one of the most direct ways to assess gut health. These tests analyze the composition and diversity of the gut microbiota, providing insights into how gut bacteria might influence kidney health.
- Gut Microbiome Profiling: Comprehensive stool tests can provide a detailed analysis of the gut microbiome, identifying beneficial bacteria as well as potentially harmful bacteria. An imbalance, or dysbiosis, in the gut microbiome, can contribute to the production of uremic toxins that burden the kidneys. Several techniques are used to evaluate the gut microbiome, with DNA-based sequencing and microbial culture being among the most common. DNA-based sequencing is more comprehensive, but it cannot differentiate between live and dead microbes. Conversely, culture-based techniques are cumbersome, but they detect living microbes. An ideal test combines DNA-based sequencing and microbial cultures such as GI Effects Comprehensive Profile by Genova Diagnostics and Doctor's Data GI360 test.
- Short-Chain Fatty Acids (SCFAs): SCFAs are produced by the fermentation of dietary fibers by gut bacteria and play a crucial role in maintaining gut health. Stool tests can measure SCFA levels, with lower levels often indicating dysbiosis. SCFAs have anti-inflammatory properties and help maintain gut barrier integrity, both of which are important for kidney health. Both tests mentioned above report SCFAs in their results.
- Pathogen Detection: Stool tests can also detect the presence of specific pathogens, such as Clostridium difficile or H. Pylori, that can cause infections and exacerbate gut inflammation, indirectly impacting kidney health. These tests are available via conventional labs.
Join us to end the kidney disease epidemic
Urine Tests for Gut Health Indicators
These tests measure the levels of various organic acids in urine, which can provide insights into several metabolic processes, including those related to gut microbiome activity. Elevated or imbalanced organic acids can indicate dysbiosis (an imbalance in gut bacteria), yeast or fungal overgrowth, and nutrient deficiencies, all of which affect gut health.
Organic acid tests contribute to gut health assessment:
- Microbial Metabolites: OATs can detect specific metabolites produced by gut bacteria and yeast. High levels of certain markers, such as D-arabinitol (a byproduct of Candida yeast) or specific bacterial metabolites, suggest overgrowth or imbalances in the gut microbiota.
- Detoxification Pathways: The test provides information about the function of the liver's detoxification processes. Certain organic acids are products of detoxification pathways, and their presence can reflect the body's ability to process microbial toxins or environmental chemicals.
- Vitamin and Nutrient Deficiencies: Some organic acids are byproducts of the body’s nutrient metabolism, including vitamins B6, B12, and CoQ10. Deficiencies in these nutrients can impair gut health and the immune system, leading to gut issues.
- Mitochondrial Function: OATs also assess energy production in cells. Since the gut lining requires adequate energy to maintain its barrier function, compromised mitochondrial function might suggest poor gut health.
Many commercially available tests can be used for this purpose, such as Organix® Comprehensive Profile and Organic Acids (OAT) by Mosaic Diagnostics. Overall, OATs can be a valuable tool for clinicians to identify underlying metabolic and microbial issues related to gut health. However, they are usually used in conjunction with other tests (like stool tests or blood work) for a more comprehensive evaluation.
Advanced Biomonitoring Techniques
Emerging technologies offer novel ways to assess gut health, providing more comprehensive data that can be integrated into kidney disease management strategies.
- Metabolomics: This approach involves analyzing metabolites in blood, urine, or stool samples to provide a snapshot of metabolic processes, many of which are influenced by the gut microbiota. Metabolomics can help identify specific metabolites linked to gut health and kidney disease, offering potential biomarkers for diagnosis and treatment. Some laboratories provide these tests but at this time they are not comprehensive enough.
- Genomic and Transcriptomic Analysis: These advanced techniques involve analyzing the genetic material of gut bacteria (genomics) and the expression of genes (transcriptomics) to understand the functional aspects of the gut microbiome. These insights can help personalize treatment plans for kidney disease patients based on their gut microbiome profile. These tests are not widely available commercially.
The Bottom Line on Assessing Gut Health for Kidney Disease Management
Given the close connection between the gut and kidneys, assessing gut health is a vital component of managing kidney disease. By using a combination of questionnaires, blood tests, stool tests, urine tests, and advanced biomonitoring techniques, healthcare providers can gain a comprehensive understanding of gut health and its impact on kidney function. This holistic approach allows for more personalized and effective management of kidney disease, potentially slowing its progression and improving patient outcomes.
Understanding Membranous Nephropathy: Causes, Symptoms, and What You Need to Know
Membranous nephropathy (MN) is a type of kidney disease that affects the glomeruli—tiny blood vessels in the kidneys responsible for filtering waste from the blood. In membranous nephropathy, the immune system mistakenly attacks these glomeruli, leading to thickening of the glomerular basement membrane (GBM). This thickening disrupts the kidney’s ability to filter blood properly, causing proteins to leak into the urine, a condition known as proteinuria. In this blog, we will discuss symptoms and causes of membranous nephropathy.
As usual, the references are in the hyperlinks.
By Majd Isreb, MD, FACP, FASN, IFMCP
What Causes Membranous Nephropathy?
The exact causes of membranous nephropathy are often unknown, making it difficult to predict who will develop the disease. However, MN can be categorized into two types based on its causes:
Primary Membranous Nephropathy
This is the most common form and occurs when the disease develops without an identifiable underlying cause. It’s thought to be an autoimmune condition where the body’s immune system mistakenly targets proteins in the GBM. In many cases, the specific antibodies responsible for this attack have been identified, such as the phospholipase A2 receptor (PLA2R) antibodies. Genetic predispositions plays a role here (see below) and even though scientists didn't identify the exact trigger it is likely because it has not been linked to MN yet.
Secondary Membranous Nephropathy
- This form occurs as a result of another condition or external factor. Common causes of secondary membranous nephropathy include:
- Infections: Hepatitis B and C, syphilis, and malaria are known to trigger MN.
- Autoimmune Diseases: Conditions like systemic lupus erythematosus (SLE) can lead to MN.
- Medications: Certain drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs), gold salts, penicillamine, and some monoclonal antibodies, have been linked to MN.
- Cancers: Some forms of cancer, particularly solid tumors like lung or colon cancer, have been associated with MN.
- Environmental Toxins: Exposure to certain toxins, such as mercury, can also cause the disease.
Join us to end the kidney disease epidemic
Symptoms of Membranous Nephropathy
The hallmark symptom of MN is proteinuria, where large amounts of protein are lost in the urine. This can lead to:
- Swelling (Edema): Most commonly in the legs, ankles, and feet, but it can also affect the face and hands.
- Foamy Urine: Due to the high levels of protein in the urine.
- Weight Gain: Often as a result of fluid retention.
- Fatigue: Caused by the loss of protein, which can affect energy levels and overall health.
- High Blood Pressure: As kidney function declines, blood pressure may increase.
Diagnosing Membranous Nephropathy
Diagnosis usually involves a combination of tests, including:
- Urinalysis: To detect protein in the urine.
- Blood Tests: To measure kidney function and levels of antibodies that may be causing MN.
- Kidney Biopsy: A small sample of kidney tissue is examined under a microscope to confirm the diagnosis and determine the extent of the damage. More recently, the commercial availability of phospholipase A2 receptor (PLA2R) antibodies tests almost eliminated the need for a kidney biopsy.
Conventional Treatment Options
The treatment for MN depends on the underlying cause, the severity of the disease, and the patient’s overall health. In cases of primary MN, treatment might focus on controlling symptoms and slowing disease progression, while secondary MN requires addressing the underlying condition.
- Medications: These may include:
- Immunosuppressants: To reduce the immune system’s attack on the kidneys.
- Corticosteroids: To decrease inflammation.
- ACE Inhibitors or ARBs: To control blood pressure and reduce proteinuria.
- Lifestyle Changes: Diet and exercise modifications may be recommended to help manage symptoms and improve kidney health.
- Treating the Underlying Cause: In cases of secondary MN, it’s crucial to address the root cause, whether it’s an infection, cancer, or medication.
Integrative Treatment Options
An integrative approach to managing MN would combine conventional medical treatments with complementary therapies to address the disease holistically. The goal would be to identify the root causes of MN, manage symptoms, slow or reverse disease progression, and support overall well-being. More on that in a future blog.
Prognosis and Long-Term Management
The outlook for people with membranous nephropathy varies widely. Some people experience spontaneous remission, where the disease improves on its own. Others may progress to chronic kidney disease (CKD) or even kidney failure, requiring dialysis or a kidney transplant. Long-term monitoring and management are essential to prevent complications and maintain kidney function.
Join us to end the kidney disease epidemic
Genetics of Membranous Nephropathy
Genetics plays a significant role in the development of membranous nephropathy, particularly in its primary form. Researchers have identified specific genetic markers that increase the risk of developing MN. One of the most notable genetic associations is with the HLA-DQA1 gene, which is involved in immune system regulation. Variations in this gene are linked to an increased likelihood of the immune system targeting the kidney’s glomerular basement membrane, leading to the thickening and damage characteristic of MN.
Additionally, the presence of autoantibodies against the phospholipase A2 receptor (PLA2R) on kidney cells is strongly correlated with genetic predisposition. These findings suggest that individuals with certain genetic backgrounds are more susceptible to the autoimmune processes that trigger membranous nephropathy, highlighting the importance of genetic factors in the disease’s onset and progression.
The Bottom Line on Membranous Nephropathy
Membranous nephropathy is a complex kidney disease with various causes and outcomes. Understanding the symptoms and causes of membranous nephropathy is crucial for managing the condition and improving quality of life. If you or someone you know is experiencing symptoms of MN, it’s important to seek medical advice early for proper diagnosis and treatment. With the right care and management, many people with MN can lead healthy lives.
September Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the September Research and News.
Proton Pump Inhibitor Use Linked to Increased Fracture Risk in Patients with Chronic Kidney Disease
A population-based observational case-control study was conducted to investigate the association between proton pump inhibitor (PPI) use and the risk of bone fractures among patients with chronic kidney disease (CKD).
Data from 12,152 patients (6,076 with fractures and 6,076 without) were analyzed using the IQVIATM Disease Analyzer database.
Multivariable logistic regression adjusted for confounding factors revealed that PPI use significantly increased the risk of fractures (odds ratio [OR] 1.68; 95% CI 1.55–1.83), particularly notable in individuals under 60 who had used PPIs for more than two years (OR 6.85; 95% CI 1.85–25.38) or those with a cumulative PPI dose above 16,000 mg (OR 4.62; 95% CI 1.87–11.44). The risk was similar between men and women.
Why is this Important?
The findings underscore the need for cautious prescription of PPIs in CKD patients, particularly among younger individuals or those with prolonged usage, as these patients already face a heightened risk of fractures.
Deprescribing PPIs in patients without a clear medical indication may significantly reduce the fracture risk in this vulnerable group, potentially decreasing morbidity and mortality associated with bone fractures in CKD patients.
This study highlights the importance of evaluating medication risks and benefits in managing CKD complications.
Dietary Fiber Intake and Its Impact on Mortality and Clinical Outcomes in Chronic Kidney Disease Patients
The study explored the association between dietary fiber intake and clinical outcomes among 3,791 participants with chronic kidney disease (CKD) from the Chronic Renal Insufficiency Cohort study.
Dietary fiber intake was assessed and participants were categorized into tertiles. Using Cox Proportional Hazards models adjusted for various factors including inflammatory markers, it was found that lower dietary fiber intake correlated with a slightly higher risk of all-cause mortality, with participants in the middle and low tertiles having a 19% and 11% greater risk of death, respectively, compared to the highest fiber intake group.
However, there were no significant associations between dietary fiber intake and adverse cardiovascular or kidney outcomes, nor were there significant associations with levels of C-reactive protein and interleukin-6.
Why is this important?
Understanding the impact of dietary fiber on CKD patients is crucial as dietary interventions are common for chronic disease management.
While the study showed a potential link between low fiber intake and increased all-cause mortality, no significant effects were observed on cardiovascular or kidney outcomes.
These findings suggest that while dietary fiber might influence survival rates, it does not appear to affect disease progression directly in CKD.
Further randomized trials are necessary to confirm these findings and potentially guide dietary recommendations for improving long-term outcomes in CKD patients.
Dietary Acid Reduction with Fruits and Vegetables Versus Sodium Bicarbonate: Impact on Kidney and Cardiovascular Health in Hypertensive Patients
This five-year, interventional, randomized control trial investigated the effects of dietary acid reduction on kidney disease progression and cardiovascular health in 153 hypertensive patients with macroalbuminuria.
Participants were assigned to receive either fruits and vegetables, oral sodium bicarbonate (NaHCO3), or continue with Usual Care.
The study found that chronic kidney disease progression was significantly slower in the fruits and vegetables or NaHCO3 groups compared to Usual Care.
Additionally, those consuming fruits and vegetables experienced greater improvements in systolic blood pressure and cardiovascular risk indices, despite lower doses of pharmacologic treatments.
Why is this important?
The study highlights the potential of non-pharmacologic interventions, specifically dietary adjustments like increased intake of fruits and vegetables, in managing hypertension and reducing the risk of chronic kidney disease and cardiovascular complications.
This emphasizes the role of diet in complementing traditional pharmacologic treatments, providing a more holistic approach to disease management in hypertensive patients.
Join us to end the kidney disease epidemic
Impact of Gestational Exposure to Systemic Glucocorticoids on Childhood Chronic Kidney Disease Risk
This retrospective cohort study, conducted in Taiwan's largest healthcare system, explored the association between gestational exposure to maternal systemic glucocorticoids and the development of chronic kidney disease (CKD) in children.
Analyzing 23,363 singleton births from 2004 to 2018, researchers utilized Cox proportional hazards models to determine risks.
Results revealed a significant association between prenatal glucocorticoid exposure and increased CKD risk in children (Adjusted Hazard Ratio, AHR, 1.69).
Notably, risks were amplified in specific subgroups, including preterm births, males, second-trimester exposures, and higher cumulative glucocorticoid dosages.
Why is this important?
Understanding the potential long-term renal risks associated with prenatal glucocorticoid exposure could influence obstetric care and postnatal monitoring, particularly in developing strategies to mitigate CKD risks in children exposed to these medications in utero.
This study highlights the need for careful consideration and potential regulation of glucocorticoid use during pregnancy to prevent adverse kidney outcomes in offspring.
Review article of the month
Cancer Drugs and Acute Kidney Injury
Cancer therapies, including traditional chemotherapies, innovative immunotherapies, and targeted treatments, have significantly improved survival rates but also pose risks of acute kidney injury (AKI).
Conventional chemotherapies like cisplatin and methotrexate are known for causing tubulointerstitial injuries, while newer immunotherapies such as immune checkpoint inhibitors and CAR-T therapies often lead to kidney immune-related adverse events, including acute interstitial nephritis and occasionally glomerular disease.
Additionally, recent targeted therapies have been linked to ‘pseudo-AKI’ by affecting renal tubular secretion of creatinine, creating misleading serum creatinine elevations, though some cases show true kidney damage.
This review discusses all these details.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Genetic Assessments for Managing Kidney Disease: A Comprehensive Approach
Kidney disease is a complex condition influenced by various factors, including genetics. Understanding the genetic underpinnings of kidney health can provide invaluable insights for early diagnosis, personalized treatment, and better management of the disease. This blog explores the different genetic assessments for managing kidney disease, discussing their relevance, applications, and the impact they can have on patient care.
Genetic Assessments for Managing Kidney Disease
By Majd Isreb, MD, FACP, FASN, IFMCP
The Role of Genetics in Kidney Disease
Genetics play a crucial role in the development and progression of kidney diseases. Conditions such as polycystic kidney disease (PKD), Alport syndrome, and congenital anomalies of the kidney and urinary tract (CAKUT) are directly linked to genetic mutations. Moreover, genetic predispositions can influence how patients respond to various treatments and their overall disease prognosis.
Join us to end the kidney disease epidemic
Genetic Assessments for Managing Kidney Disease: Key Methods
Genetic Testing for Inherited Kidney Disorders
Genetic testing is a powerful tool for identifying specific mutations associated with inherited kidney disorders. By analyzing an individual's DNA, healthcare providers can determine the presence of mutations that predispose them to certain kidney diseases.
- Polycystic Kidney Disease (PKD): Genetic testing can identify mutations in the PKD1 and PKD2 genes, which are responsible for the majority of autosomal dominant polycystic kidney disease (ADPKD) cases. Early detection through genetic testing allows for proactive management of the disease, including monitoring kidney function and controlling blood pressure.
- Alport Syndrome: This genetic disorder, which affects the kidneys, ears, and eyes, is caused by mutations in the COL4A3, COL4A4, or COL4A5 genes. Genetic testing can confirm a diagnosis, allowing for early intervention to slow the progression of kidney damage.
- Congenital Anomalies of the Kidney and Urinary Tract (CAKUT): Genetic assessments can identify mutations linked to CAKUT, helping in the diagnosis and management of these congenital conditions that can lead to chronic kidney disease (CKD) later in life.
Next-Generation Sequencing (NGS) for Comprehensive Genetic Analysis
Next-generation sequencing (NGS) is an advanced genetic testing method that allows for the simultaneous analysis of multiple genes. This technology has revolutionized the field of genetics by providing comprehensive data on genetic mutations, including those related to kidney health.
- Gene Panels: NGS can be used to analyze specific gene panels related to kidney diseases. For instance, a nephropathy gene panel can include genes associated with glomerulopathies, tubulopathies, and other renal conditions. This targeted approach helps in identifying the genetic basis of kidney disease in patients with unclear diagnoses.
- Whole Exome Sequencing (WES): WES focuses on sequencing the exome, which is the protein-coding region of the genome. This method can uncover rare genetic variants that may contribute to kidney disease, providing insights that may not be evident through traditional testing methods.
- Whole Genome Sequencing (WGS): WGS offers the most comprehensive analysis by sequencing the entire genome. While this method is more expensive and complex genetic assessement for managing kidney disease, it can identify non-coding variants and structural changes in the genome that could affect kidney health.
Pharmacogenomic Testing: Tailoring Treatment Based on Genetics
Pharmacogenomics involves studying how an individual's genetic makeup affects their response to drugs. In the context of kidney health, pharmacogenomic testing can help in personalizing treatment plans, reducing adverse drug reactions, and optimizing therapeutic outcomes.
- ACE Inhibitors and ARBs: These drugs are commonly used to manage hypertension and protect kidney function in patients with CKD. Pharmacogenomic testing can identify genetic variants that affect how patients metabolize these medications, allowing for dose adjustments or alternative therapies.
- Immunosuppressants: In patients with kidney transplants, immunosuppressive drugs are essential to prevent rejection. Genetic testing can predict how patients will respond to these drugs, helping to balance efficacy and toxicity, and reducing the risk of adverse effects.
- Warfarin: Patients with CKD are at higher risk of developing blood clots and may require anticoagulant therapy. Genetic variants in the CYP2C9 and VKORC1 genes can influence how patients respond to warfarin, a commonly prescribed anticoagulant. Pharmacogenomic testing ensures that the correct dosage is administered to minimize bleeding risks.
Preimplantation Genetic Testing (PGT) for Inherited Kidney Disease
For couples with a known risk of passing on genetic kidney diseases, preimplantation genetic testing (PGT) offers a way to ensure that their offspring are free from specific genetic mutations.
- PGT for Monogenic Disorders (PGT-M): This form of PGT is used to detect single-gene mutations, such as those causing PKD or Alport syndrome, in embryos created through in vitro fertilization (IVF). By selecting embryos without the disease-causing mutations, parents can reduce the risk of transmitting kidney disease to their children.
- Ethical Considerations: While PGT offers significant benefits, it also raises ethical concerns, particularly regarding the selection of embryos and the potential for designer babies. It is essential for healthcare providers to guide patients through these decisions with sensitivity and respect for their values.
Family History and Pedigree Analysis
While advanced genetic testing is crucial, traditional methods like family history and pedigree analysis remain valuable tools in assessing the risk of kidney disease. These methods involve documenting the occurrence of kidney disease and related conditions within a family, helping to identify patterns that may indicate a genetic predisposition.
- Identifying At-Risk Individuals: By analyzing family history, healthcare providers can identify individuals who may benefit from genetic testing, early screening, and preventive measures.
- Guiding Genetic Counseling: Pedigree analysis is also essential in genetic counseling, helping families understand their risk of inherited kidney diseases and make informed decisions about their health.
The Bottom Line on Genetic Assessement for Managing Kidney Disease
Genetic assessments are integral to managing kidney disease, offering insights that enable early diagnosis, personalized treatment, and improved patient outcomes. From traditional methods of genetic assessment for managing kidney disease like family history analysis to advanced techniques like next-generation sequencing and pharmacogenomics, these tools provide a comprehensive approach to understanding and mitigating the genetic risks associated with kidney disease. By embracing these genetic assessments, healthcare providers can offer more targeted and effective care, ultimately improving the lives of individuals with kidney health concerns.
Five Potential IgA Nephropathy Triggers
IgA nephropathy, also known as Berger’s disease, is a chronic kidney disorder where immunoglobulin A (IgA) builds up in the kidneys, leading to inflammation and potential kidney damage. This autoimmune condition can vary widely in its severity, with some individuals experiencing only mild symptoms while others progress to more severe kidney disease. Understanding the factors that can trigger or exacerbate IgA nephropathy is crucial for managing the condition and preventing further kidney damage. In this blog, we will explore five IgA nephropathy triggers, including infections, food sensitivities, and more.
As usual, the references are in the hyperlinks.
By Majd Isreb, MD, FACP, FASN, IFMCP
IgA Nephropathy Triggers
Understanding IgA nephropathy
IgA nephropathy is an autoimmune disorder predominantly affecting mesangial cells. The disease process begins when the immune system erroneously produces abnormal IgA proteins. These proteins, perceived as foreign, instigate an immune response. Immune complexes formed in this process accumulate in the kidneys, attaching to mesangial cells, leading to inflammation and kidney tissue damage.
The ‘four-hit’ hypothesis of IgAN
The pathogenesis of IgAN is encapsulated in the “four-hit hypothesis,” which posits:
- Elevated levels of abnormal IgA1 (galactose-deficient IgA1 or gd-IgA1)
- Autoantibody production against this abnormal IgA1
- Formation of immune complexes involving these autoantibodies and CD89
- Deposition of these complexes in the glomerular mesangium, culminating in kidney damage
Significant to IgAN is dimeric IgA1, predominantly originating from the gut’s mucosa-associated lymphoid tissue (MALT). In fact, the site of gd-IgA1 production is presumed to be the Peyer’s patches of the gut and mesenteric lymph nodes.
The exact reason for abnormal IgA1 production remains unknown, but genetic and environmental factors likely contribute. This review article offers detailed insights into IgAN’s innate and adaptive immune mechanisms. It is freely accessible.
Five IgA Nephropathy Triggers
Infections: A Common IgA Nephropathy Trigger
One of the most well-known triggers for IgA nephropathy is infections, particularly those affecting the respiratory tract. Upper respiratory infections, such as the common cold or strep throat, can lead to an increased production of abnormal IgA antibodies. In individuals with a predisposition to IgA nephropathy, these antibodies can deposit in the kidneys, causing inflammation and damage. This response may occur within days or weeks following the infection, highlighting the importance of closely monitoring kidney function during and after an illness.
In addition to respiratory infections, gastrointestinal infections have also been linked to IgA nephropathy flare-ups. Bacterial or viral infections in the gut can similarly stimulate an overproduction of abnormal IgA, which can then accumulate in the kidneys. Dysbiosis in the gut can also be a trigger to IgA Nephropathy in individuals with predispostion.
Food Sensitivities: Hidden IgA Nephropathy Triggers
While not as widely recognized as infections, food sensitivities can also trigger IgA nephropathy in some individuals. Certain foods, particularly those that provoke an immune response, may lead to the formation and deposition of IgA in the kidneys. Gluten, found in wheat, barley, and rye, is one of the most commonly implicated food sensitivities in individuals with autoimmune conditions, including IgA nephropathy.
Soy, egg white, milk protein and oat have also been associated with kidney inflammation in sensitive individuals. Identifying and eliminating trigger foods from the diet can be a crucial step in managing IgA nephropathy. Working with a healthcare provider or nutritionist to identify food sensitivities through elimination diets or testing may help reduce flare-ups and protect kidney function.
Join us to end the kidney disease epidemic
Stress: An Overlooked Trigger for IgA Nephropathy
Stress is often an overlooked factor in chronic diseases, but it can have a significant impact on autoimmune conditions like IgA nephropathy. Both physical and emotional stress can alter immune system function, potentially leading to increased production of abnormal IgA in predisposed individuals leading to subsequent kidney damage. Stress-induced flare-ups can occur due to various reasons, such as surgery, trauma, or even psychological stressors like anxiety or depression.
Managing stress through lifestyle modifications, such as regular exercise, mindfulness practices, and adequate sleep, can be beneficial for individuals with IgA nephropathy. Additionally, stress management strategies can help improve overall well-being, making it easier to cope with the challenges of living with a chronic kidney condition.
Environmental Factors: Triggers Lurking in Your Surroundings
Environmental factors, including exposure to certain chemicals and pollutants, can also act as triggers for IgA nephropathy. For example, exposure to air pollution, particularly fine particulate matter, has been linked to increased kidney inflammation and dysfunction in susceptible individuals. Similarly, contact with certain chemicals, such as those found in household cleaners or industrial products, may exacerbate IgA nephropathy.
Reducing exposure to environmental triggers can be challenging, but taking steps to minimize contact with pollutants and chemicals is essential. This might include using air purifiers, avoiding smoking or secondhand smoke, and choosing natural or organic cleaning products. Being mindful of environmental factors can help reduce the risk of flare-ups and protect kidney health.
Medications: Unintentional IgA Nephropathy Triggers
Certain medications can unintentionally trigger or worsen IgA nephropathy. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or naproxen, are commonly used for pain relief but can cause kidney inflammation and damage, particularly in individuals with pre-existing kidney conditions. They are also linked to dysbiosis. Overuse or prolonged use of these medications may lead to a worsening of IgA nephropathy symptoms.
Other medications, including some antibiotics can also lead to dysbiosis which in-turn can trigger flare-ups. It’s important for individuals with IgA nephropathy to consult their healthcare providers before starting any new medications and to discuss potential risks. Adjusting dosages or exploring alternative treatments may be necessary to protect kidney health.
The Bottom Line on IgA Nephropathy Triggers
IgA nephropathy is a complex condition with various triggers that can activate or exacerbate the disease. By understanding and identifying these triggers—such as infections, food sensitivities, stress, environmental factors, and certain medications—individuals with IgA nephropathy can take proactive steps to manage their condition and protect their kidney health. If you or a loved one has been diagnosed with IgA nephropathy, it’s essential to work closely with healthcare providers to develop a personalized management plan that addresses these triggers and reduces the risk of kidney damage.
The Impact of Forever Chemicals on Kidney Disease Prevalence and Progression
Forever chemicals, scientifically known as per- and polyfluoroalkyl substances (PFAS), are a group of man-made chemicals that have been used in various industries worldwide since the 1940s. Known for their persistence in the environment and human body, these chemicals have been linked to a host of health issues, including kidney disease. This blog explores the effects of forever chemicals on the prevalence of kidney disease and the progression of various kidney diseases, highlighting the urgent need for increased awareness and regulatory measures.
By Majd Isreb, MD, FACP, FASN, IFMCP
What Are Forever Chemicals?
Forever chemicals are characterized by their strong carbon-fluorine bonds, making them resistant to degradation. This persistence leads to their accumulation in the environment, wildlife, and human tissues. PFAS are found in everyday products such as non-stick cookware, water-repellent clothing, and firefighting foams, resulting in widespread exposure. Studies have shown that exposure to these chemicals can lead to adverse health effects, including kidney disease.
Forever Chemicals and Kidney Disease Prevalence
Research indicates that exposure to forever chemicals can increase the prevalence of kidney disease. A study published in the American Journal of Epidemiology found that individuals with higher levels of PFAS in their blood had an increased risk of developing chronic kidney disease (CKD). The study suggests that PFAS may contribute to kidney damage by disrupting endocrine functions and promoting inflammation. Moreover, communities living near industrial sites where PFAS are produced or used are at a higher risk of exposure and subsequent kidney disease.
Join us to end the kidney disease epidemic
Mechanisms of Kidney Damage
The mechanisms by which forever chemicals induce kidney damage are complex and multifaceted. PFAS can interfere with the normal functioning of the kidneys by altering hormone levels, leading to increased blood pressure and reduced kidney filtration efficiency. Additionally, these chemicals can cause oxidative stress and inflammation, further contributing to kidney injury. Studies have also suggested that PFAS may disrupt the function of the renal proximal tubules, which are crucial for filtering waste from the blood.
Progression of Kidney Disease and Forever Chemicals
Forever chemicals not only increase the prevalence of kidney disease but also exacerbate the progression of existing CKD. Patients with CKD exposed to high levels of PFAS may experience accelerated disease progression, leading to end-stage renal disease (ESRD). This is particularly concerning as ESRD requires dialysis or kidney transplantation, significantly impacting the quality of life and healthcare costs.
Nutrition and Forever Chemicals
Dietary Sources of PFAS
One of the primary routes of PFAS exposure is through diet. These chemicals can contaminate food and water supplies, leading to ingestion by humans. Foods like fish, dairy products, and certain packaged foods are known to contain PFAS, particularly if they are sourced from contaminated areas or packaged using materials containing these chemicals.
Nutritional Interventions
Proper nutrition can help mitigate some of the adverse effects of forever chemicals on kidney health. A diet rich in antioxidants, such as fruits and vegetables, can combat oxidative stress caused by PFAS. Omega-3 fatty acids, found in fish and flaxseed, have anti-inflammatory properties that may protect the kidneys from PFAS-induced damage. Additionally, adequate hydration can support kidney function and help in the excretion of toxins.
Dietary Patterns and Risk Modulation
Adopting healthy dietary patterns, such as the Mediterranean diet, which emphasizes whole foods, healthy fats, and minimal processed foods, can reduce the overall burden of PFAS. Conversely, diets high in processed foods, which often come in PFAS-containing packaging, can increase exposure and exacerbate kidney damage.
Join us to end the kidney disease epidemic
Genetics and Forever Chemicals
Genetic Susceptibility
Genetics plays a significant role in an individual’s susceptibility to the harmful effects of PFAS. Certain genetic polymorphisms can affect how the body metabolizes and excretes these chemicals. For instance, variations in genes related to renal function and detoxification pathways can influence the extent of kidney damage caused by PFAS exposure.
Genetic Research
Recent studies have identified specific genetic markers associated with increased vulnerability to PFAS. For example, polymorphisms in genes like PPARA (peroxisome proliferator-activated receptor alpha) and other genes involved in lipid metabolism and inflammation have been linked to heightened sensitivity to PFAS. Understanding these genetic factors can aid in identifying at-risk populations and developing personalized intervention strategies.
Family History and Kidney Disease
A family history of kidney disease can also indicate a genetic predisposition that may exacerbate the effects of PFAS exposure. Individuals with a genetic background of renal issues should be particularly cautious about PFAS exposure and consider regular kidney function monitoring.
Vulnerable Populations
Certain populations are more vulnerable to the effects of forever chemicals on kidney disease. These include individuals with pre-existing kidney conditions, the elderly, and those with compromised immune systems. Additionally, socioeconomically disadvantaged communities often face higher exposure levels due to proximity to industrial sites and inadequate access to clean water. This highlights the need for targeted interventions to protect these high-risk groups.
Mitigating the Impact of Forever Chemicals
Efforts to mitigate the impact of forever chemicals on kidney health include both individual and collective actions. On a personal level, reducing the use of products containing PFAS and advocating for regular health check-ups can help manage exposure risks. On a broader scale, supporting legislation aimed at regulating PFAS and funding research on safer alternatives is vital. Healthcare providers should also be informed about the potential risks of PFAS to better guide patient care and preventive strategies.
Integrating Nutrition and Genetics in Public Health Strategies
Personalized Nutrition Plans
Combining knowledge of nutrition and genetics can lead to personalized nutrition plans aimed at minimizing the impact of PFAS on kidney health. For example, individuals with a genetic predisposition to kidney disease might benefit from a diet specifically tailored to enhance renal function and reduce oxidative stress.
Community Education
Educating communities about the role of nutrition and genetics in PFAS exposure and kidney health can empower individuals to make informed choices. Public awareness campaigns should highlight the importance of dietary habits and genetic predispositions in managing the risks associated with forever chemicals.
Regulatory and Policy Implications
The growing body of evidence linking forever chemicals to kidney disease underscores the urgent need for stringent regulatory measures. A recent study, for example, showed that 45% of tap water in the US is contaminated with PFAS. Therefore, policies aimed at reducing PFAS production and use, as well as monitoring and mitigating exposure, are crucial. Governments and regulatory bodies must enforce stricter limits on PFAS levels in drinking water and push for the development of safer alternatives. Public awareness campaigns are also essential to educate individuals about the risks and preventive measures related to forever chemicals.
The Bottom Line on Forever Chemicals and Kidney Disease
The evidence linking forever chemicals to the prevalence and progression of kidney disease is compelling and calls for immediate action. As we continue to uncover the far-reaching impacts of PFAS, it is imperative to adopt comprehensive strategies to reduce exposure, protect vulnerable populations, and promote kidney health. By understanding and addressing the risks associated with forever chemicals, we can work towards a healthier future free from the burden of kidney disease.
August Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the August Research and News.
Sodium Bicarbonate in CKD with Metabolic Acidosis: Benefits and Risks Unveiled
A meta-analysis of fourteen randomized controlled trials involving 2,037 patients with chronic kidney disease (CKD) and metabolic acidosis (MA) explored the efficacy of sodium bicarbonate treatment.
The findings revealed significant improvements in estimated glomerular filtration rate (eGFR) and reductions in hospitalization rates.
Additionally, patients receiving sodium bicarbonate demonstrated an increase in mid-arm muscle circumference (MAMC), suggesting enhanced muscle mass.
However, the treatment was associated with elevated systolic blood pressure (SBP), with no notable difference in all-cause mortality observed.
Why is this important?
This study underscores the potential of sodium bicarbonate to mitigate kidney function decline and improve muscle mass in CKD patients with MA, which could significantly enhance their quality of life.
However, the associated risk of elevated blood pressure highlights the need for careful monitoring and tailored therapeutic strategies.
The findings call for further research, particularly given the methodological limitations such as the lack of double-blinded designs and inconsistencies in control group definitions across the included studies, to confirm these outcomes and refine treatment protocols.
Link Between Bowel Movement Frequency and Kidney Health: Insights from Microbial Metabolites
Research has highlighted the profound impact of bowel movement frequency (BMF) on the gut microbiota, revealing connections with systemic health and specific conditions such as chronic kidney disease.
Analyzing data from healthy adults, the study found that variations in BMF are linked to changes in gut microbial communities and blood metabolites, reflecting differences in inflammation, heart, liver, and kidney function.
Particularly notable is the association of constipation with increased levels of the toxin 3-indoxyl sulfate (3-IS), which adversely affects kidney function.
Why is this important?
This study illuminates how irregular BMF, particularly constipation, can lead to the buildup of harmful microbial toxins in the blood, contributing to organ dysfunction and potentially precipitating chronic diseases associated with aging.
The findings underscore the importance of maintaining regular bowel movements through dietary and lifestyle choices to prevent the long-term health consequences of microbial imbalances in the gut.
This could lead to preventive strategies in clinical practice, enhancing early intervention efforts for at-risk populations.
Impact of Reducing Processed and Unprocessed Red Meat on Health Outcomes in the USA
A microsimulation study explored the potential health benefits of reducing processed and unprocessed red meat consumption in the U.S. adult population.
By analyzing dietary data from the National Health and Nutrition Examination Survey and applying risk models, researchers predicted significant reductions in type 2 diabetes, cardiovascular disease, colorectal cancer, and all-cause mortality with decreased meat consumption.
Specifically, a 30% reduction in processed meat intake could significantly lower occurrences of these conditions, with even greater benefits observed when both meat types were reduced.
Why is this important?
This study highlights the substantial public health benefits of moderating processed and unprocessed red meat intake.
By quantifying the potential reductions in major chronic diseases and mortality, it provides a compelling case for dietary guidelines and public health strategies aimed at reducing meat consumption.
The findings support initiatives to promote healthier eating patterns, potentially leading to significant improvements in population health and a decrease in healthcare costs associated with chronic diseases.
Join us to end the kidney disease epidemic
Sleep Duration and Risk of Chronic Kidney Disease: Systematic Review and Meta-Analysis
This systematic review and meta-analysis investigated the association between sleep duration and the risk of developing chronic kidney disease (CKD).
Analyzing data from 42 studies involving over 2.6 million participants, the study found that both excessively short (≤7 hours) and long (≥8 hours) sleep durations significantly increase the risk of CKD compared to the recommended 7-8 hours.
These findings were consistent across various demographics and did not change significantly with variations in age, gender, geographical region, or BMI.
Why is this important?
Understanding the impact of sleep duration on CKD risk underscores the importance of sleep management in preventing kidney disease.
By highlighting the risks associated with both insufficient and excessive sleep, this study provides a basis for healthcare professionals to recommend balanced sleep habits as a preventive measure against CKD, potentially reducing the burden of kidney disease through manageable lifestyle changes.
Review article of the month
Exercise and Cognitive Function in CKD
Individuals with chronic kidney disease (CKD) are more susceptible to cognitive decline. Exercise is believed to enhance cognitive abilities. To evaluate the effectiveness and potential adverse effects of exercise on cognitive function, this systematic review and meta-analysis focused on randomized controlled trials (RCTs) involving people with CKD.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Five Genetic Kidney Diseases You Should Know About
Kidney diseases often arise from a mix of genetic, environmental, and lifestyle factors. However, some kidney conditions are purely genetic, meaning they are caused by mutations or abnormalities in specific genes. Understanding these genetic kidney diseases is crucial for early diagnosis, appropriate management, and sometimes even prevention. Here are five genetic kidney diseases you should know about.
By Majd Isreb, MD, FACP, FASN, IFMCP
There are many genetic kidney disease but here we will discuss the most common five:
Five Genetic Kidney Diseases
Polycystic Kidney Disease (PKD)
Polycystic Kidney Disease (PKD) is one of the most common genetic kidney disorders. It is characterized by the growth of numerous cysts in the kidneys, which can lead to kidney failure.
Prevalence:
- Autosomal Dominant Polycystic Kidney Disease (ADPKD): Affects approximately 1 in 400 to 1,000 people worldwide.
- Autosomal Recessive Polycystic Kidney Disease (ARPKD): A rarer form, affecting approximately 1 in 20,000 live births.
Symptoms:
- High blood pressure
- Back or side pain
- Blood in the urine
- Kidney stones
- Urinary tract infections
Management: Unfortunately there is no cure for PKD, however many lifestyle modifications can help slow the progression of this kidney disease. Ketogenic diet may also slow the progression of PKD. Other treatments focus on managing symptoms and slowing progression. These may include blood pressure control, pain management, and, in severe cases, dialysis or kidney transplantation.
Alport Syndrome
Alport Syndrome is a genetic condition that affects the kidneys, hearing, and eyes. It results from mutations in the COL4A3, COL4A4, or COL4A5 genes, which are essential for the structure and function of the kidney's filtering units (glomeruli).
Prevalence: Affects approximately 1 in 5,000 to 10,000 people worldwide.
Join us to end the kidney disease epidemic
Symptoms:
- Blood in the urine (hematuria)
- Protein in the urine (proteinuria)
- Progressive loss of kidney function
- Hearing loss
- Eye abnormalities
Management: Management focuses on slowing kidney damage, controlling blood pressure, and addressing hearing and vision issues. In advanced stages, dialysis or kidney transplantation may be necessary.
Fabry Disease
Fabry Disease is a rare genetic disorder resulting from a deficiency of the enzyme alpha-galactosidase A, caused by mutations in the GLA gene. This leads to the buildup of a fatty substance called globotriaosylceramide in the kidneys and other organs.
Prevalence: Affects approximately 1 in 40,000 males and 1 in 20,000 females worldwide.
Symptoms:
- Pain and burning sensations in the hands and feet
- Decreased ability to sweat
- Heat intolerance
- Gastrointestinal issues
- Progressive kidney damage
- Heart problems
Management: Enzyme replacement therapy (ERT) is the primary treatment for Fabry Disease, helping to reduce the accumulation of globotriaosylceramide. Symptom management and regular monitoring of kidney function are also essential.
Nephronophthisis
Nephronophthisis is a group of genetic disorders that lead to chronic kidney disease in children and young adults. It is caused by mutations in various genes, including NPHP1, NPHP2, and others, which affect the structure and function of kidney tubules.
Prevalence: Affects approximately 1 in 50,000 to 100,000 people worldwide.
Symptoms:
- Excessive thirst and urination
- Anemia
- Growth retardation
- Progressive loss of kidney function
Management: Sadly no cure for nephronophthisis. Treatment focuses on managing symptoms and slowing the progression of kidney disease. In advanced cases, dialysis or kidney transplantation may be required.
Cystinosis
Cystinosis is another rare genetic disorder caused by mutations in the CTNS gene, leading to the accumulation of the amino acid cystine within cells, particularly in the kidneys.
Prevalence: Affects approximately 1 in 100,000 to 200,000 live births worldwide.
Symptoms:
- Fanconi syndrome (a type of kidney dysfunction)
- Excessive thirst and urination
- Growth retardation
- Photophobia (sensitivity to light)
- Muscle weakness
Management: Cystine-depleting agents, such as cysteamine, are used to reduce cystine levels in the body. Early and ongoing treatment is essential to manage symptoms and prevent complications.
The Bottom Line on Five Genetic Kidney Diseases
Genetic kidney diseases pose unique challenges due to their hereditary nature and often early onset. Awareness, early diagnosis, and appropriate management are crucial to improving outcomes for individuals with these conditions. Genetic counseling is also recommended for affected families to understand the risks and implications of these disorders.
By staying informed about these five genetic kidney diseases, individuals and healthcare providers can work together to ensure timely and effective care, ultimately improving the quality of life for those affected.
Mental Health and Kidney Disease: The Psychological Aspects and the Role of Mental Health Support in Comprehensive Care
Living with kidney disease is a challenging journey that extends beyond physical health, significantly impacting mental well-being. The interplay between kidney disease and mental health is profound, often creating a cycle where each condition exacerbates the other. This blog explores the psychological aspects of living with kidney disease and the crucial role mental health support plays in comprehensive care. We will explore the link between mental health and kidney disease and, as usual, the references are in the hyperlinks.
By Majd Isreb, MD, FACP, FASN, IFMCP
Mental Health and Kidney Disease
The Psychological Impact of Kidney Disease
Anxiety and Depression
Patients with kidney disease often experience high levels of anxiety and depression. The chronic nature of the illness, frequent medical appointments, dietary restrictions, and the potential for progression to end-stage renal disease (ESRD) contribute to a heightened sense of uncertainty and fear. Studies have shown that depression is prevalent in 20-30% of patients with chronic kidney disease (CKD), significantly higher than the general population. On the other hand, anxiety symptoms are present in as many as 43% of CKD patients.
Emotional Stress and Coping Challenges
The emotional stress of managing a chronic illness can be overwhelming. Patients may struggle with the loss of independence, changes in body image, and the financial burden of ongoing treatment. Coping with these changes requires substantial emotional resilience, which can be difficult to maintain without adequate support.
Social Isolation
Kidney disease can lead to social isolation due to physical limitations, fatigue, and the need for frequent medical treatments. This isolation can further contribute to feelings of loneliness and depression, creating a vicious cycle that deteriorates mental health. It is also associated with faster decline in kidney function.
Join us to end the kidney disease epidemic
The Role of Mental Health Support in Comprehensive Care
Psychological Counseling and Therapy
Integrating psychological counseling and therapy into the treatment plan for kidney disease patients is essential. Cognitive-behavioral therapy (CBT) has been effective in helping patients manage depression and anxiety by changing negative thought patterns and improving coping strategies.
Support Groups and Peer Support
Connecting with others who are experiencing similar challenges can provide immense relief and support. Support groups, whether in-person or online, offer a safe space for patients to share their experiences, learn from others, and feel less alone. Peer support can be incredibly empowering and help patients build a strong social network.
Mind-Body Practices
Mind-body practices such as mindfulness meditation, yoga, and relaxation techniques can reduce stress, improve mood, and enhance overall well-being. These practices help patients develop a more positive outlook and better manage the emotional toll of their condition.
Integrative Care Models
Integrative care models that combine physical and mental health services ensure a holistic approach to treatment. Multidisciplinary teams, including nephrologists, psychologists, social workers, and dietitians, can work together to address the comprehensive needs of kidney disease patients.
Patient Education and Empowerment
Educating patients about the mental health aspects of kidney disease and the importance of seeking help is crucial. Empowering patients with knowledge about their condition and available resources encourages proactive management and improves outcomes.
Regular Mental Health Screening
Routine mental health screenings should be part of the standard care protocol for kidney disease patients. Early detection of psychological issues allows for timely intervention, preventing further deterioration of mental health.
The Bottom Line about Mental Health and Kidney Disease
The psychological aspects of living with kidney disease are complex and multifaceted, requiring a comprehensive approach to care that addresses both physical and mental health needs. By integrating mental health support into the treatment plans, healthcare providers can improve the quality of life for kidney disease patients, helping them navigate their journey with greater resilience and hope. As we continue to advance our understanding of the connection between mental health and kidney disease, it is essential to foster an environment of holistic care that prioritizes the well-being of the whole person.
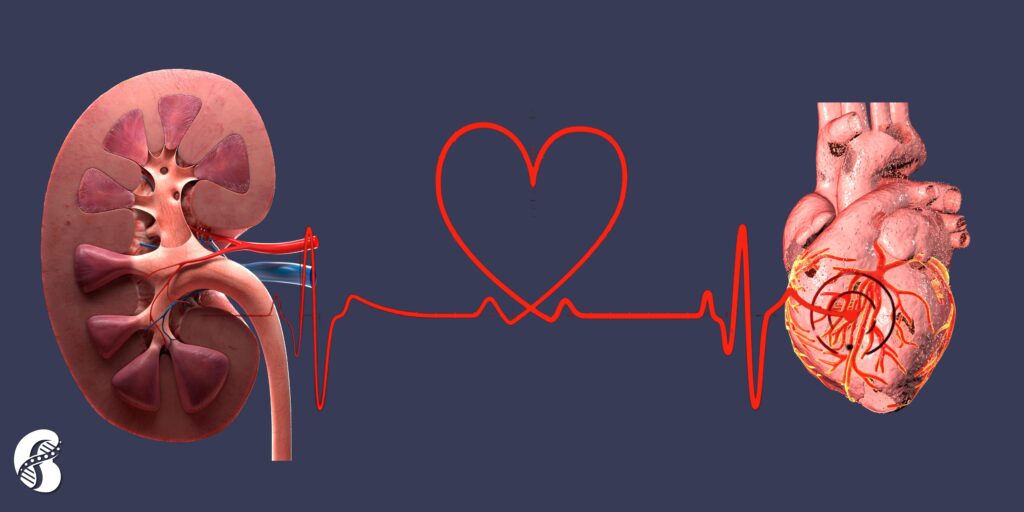
Understanding Cardiovascular-Kidney-Metabolic Syndrome: An Integrated Approach to Health
In the ever-evolving landscape of medical science, the American Heart Association (AHA) has introduced a pivotal concept known as cardiovascular-kidney-metabolic syndrome (CKM). This term encapsulates the intricate interplay between heart disease, kidney disease, diabetes, and obesity. Understanding and addressing CKM syndrome is essential for improving patient outcomes and advancing public health strategies.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Genesis of Cardiovascular-Kidney-Metabolic Syndrome
CKM syndrome represents a holistic approach to recognizing and managing the interconnectedness of cardiovascular, renal, and metabolic health issues. Traditionally, these conditions were treated in isolation, but the AHA's integrated perspective underscores the necessity of a more comprehensive treatment approach. The need for this paradigm shift has become evident as the rates of cardiovascular mortality, which had been declining, now appear to be plateauing and even rising in the post-pandemic era.
Stages of Cardiovascular-Kidney-Metabolic Syndrome
The AHA has delineated CKM syndrome into five distinct stages, each with specific characteristics and recommended interventions:
Stage 0: No CKM Risk Factors
- At this stage, the goal is prevention. Individuals should maintain ideal health by following the AHA's Life’s Essential 8 recommendations, which include healthy eating, physical activity, optimal sleep habits, avoiding nicotine, and maintaining ideal weight, blood pressure, blood sugar, and cholesterol levels.
Join us to end the kidney disease epidemic
Stage 1: Excess Body Fat and Impaired Glucose Tolerance
- This stage involves the accumulation of excess body fat, particularly abdominal obesity, and/or prediabetes. Interventions focus on lifestyle changes such as improved diet and increased physical activity, aiming for at least 5% weight loss. Regular screening every two to three years is recommended per AHA.
Stage 2: Metabolic Risk Factors and Kidney Disease
- Individuals at this stage may have type 2 diabetes, high blood pressure, high triglycerides, or chronic kidney disease. The goal is to manage these conditions to prevent progression to cardiovascular disease and kidney failure. Medications to control blood pressure, blood sugar, and cholesterol are often necessary. However, lifestyle modifications should continue to be implemented and prioritized.
Stage 3: Subclinical Cardiovascular Disease
- This stage involves early signs of cardiovascular disease without obvious symptoms, in individuals with metabolic risk factors or kidney disease. The focus is on intensifying treatment to prevent symptomatic cardiovascular disease and kidney failure, potentially including coronary artery calcium screening to guide treatment decisions.
Stage 4: Symptomatic Cardiovascular Disease
- Divided into 4a (without kidney failure) and 4b (with kidney failure), this stage includes conditions such as coronary heart disease, heart failure, and stroke. Individualized treatment plans are crucial at this stage, often involving more aggressive medical and lifestyle interventions.
Preventive and Therapeutic Approaches to the Cardiovascular-Kidney-Metabolic Syndrome
Early Identification and Intervention
The AHA's advisory highlights the importance of early identification and intervention. For instance, individuals in Stage 0 should be screened every three to five years to monitor blood pressure, triglycerides, HDL cholesterol, and blood sugar levels. Such proactive measures can significantly reduce the risk of developing CKM syndrome.
Lifestyle Modifications
At every stage, lifestyle modifications play a crucial role. Encouraging healthy eating, regular physical activity, and weight management can help mitigate the progression of CKM syndrome. In Stages 1 and 2, these changes are particularly impactful, with potential regression to lower stages of the syndrome.
Medical Treatments
In more advanced stages, medical treatments become essential. The use of SGLT2 inhibitors and GLP-1 receptor agonists in Stage 2, for example, can protect kidney function and reduce the risk of heart failure. These medications, combined with lifestyle changes, offer a comprehensive approach to managing CKM syndrome.
Addressing Social Determinants of Health
The AHA also emphasizes the importance of addressing social determinants of health. Disparities in healthcare access and socioeconomic factors significantly influence the prevalence and management of CKM syndrome. By integrating social determinants into the treatment paradigm, healthcare providers can better tailor interventions to meet the needs of diverse populations.
The Bottom Line on the Cardiovascular-Kidney-Metabolic Syndrome
Cardiovascular-kidney-metabolic syndrome represents a significant advancement in understanding the interconnectedness of heart, kidney, and metabolic health. By adopting an integrated approach, emphasizing early intervention, lifestyle modifications, and appropriate medical treatments, the AHA aims to improve outcomes for individuals affected by this complex syndrome. As we continue to unravel the intricacies of CKM syndrome, the focus on holistic health and preventive care will be paramount in shaping the future of medical practice.
July Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the July Research and News.
Higher Beta-Hydroxybutyrate Levels Linked to Slower Decline in Kidney Function in ADPKD Patients
Research within the Developing Intervention Strategies to Halt Progression of ADPKD (DIPAK) cohort, involving 670 participants, explored the impact of endogenous beta-hydroxybutyrate (BHB) on the progression of autosomal dominant polycystic kidney disease (ADPKD).
The study aimed to address a gap in understanding how ketone bodies, specifically BHB, influence ADPKD, known for its reliance on glucose and poor utilization of other energy sources like ketones.
Data analysis excluded participants with diabetes, those on specific disease-modifying drugs, non-fasting individuals, or those with missing BHB data, resulting in 521 eligible participants.
These participants had a median BHB level of 94 µmol/L and demonstrated that while BHB levels did not correlate with immediate kidney function as measured by glomerular filtration rate (eGFR) or kidney volume, higher BHB levels were longitudinally associated with a slower decline in kidney function.
Specifically, each doubling of BHB concentration correlated with significant improvements in annual eGFR rates, suggesting potential therapeutic benefits of increasing BHB levels in ADPKD management.
Why is this important?
This study underscores the potential of targeting metabolic pathways in ADPKD treatment, specifically through elevating beta-hydroxybutyrate levels, which could notably decelerate kidney function decline.
This finding opens avenues for metabolic interventions in ADPKD, shifting focus towards dietary strategies to elevate ketone bodies, such as ketogenic diet, thereby possibly offering a new, non-invasive method to manage and potentially slow the progression of the disease.
Impact of Early Life Famine Exposure on Kidney Function Decline in Later Life: Evidence from the Great Chinese Famine
This retrospective analysis utilized data from 8,828 participants of the CHARLS study to explore the long-term impact of the Great Chinese Famine (1959-1962) on kidney function, measured by the decline in glomerular filtration rate (eGFR).
Participants were divided into groups based on their exposure periods: fetal-exposed (1959-1962), childhood-exposed (1949-1958), adolescence/adult-exposed (1912–1948), and non-exposed (1963–1989).
Results showed a significant association between famine exposure and reduced eGFR, particularly pronounced in those exposed during adolescence/adulthood.
Adjustments for demographics, health behaviors, and chronic conditions did not alter these findings, suggesting a robust link between early life famine exposure and subsequent kidney function decline.
Why is this important?
Understanding the long-term effects of famine exposure on kidney health is crucial for public health planning and intervention, especially in aging populations with historical exposures to extreme conditions.
This study highlights the importance of early life conditions in determining kidney outcomes likely via epigenetic changes. It supports the need for targeted healthcare strategies to mitigate the long-term adverse effects of such exposures on kidney health.
Critical Role of Thyroid Hormone Levels in Progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
This study investigated the impact of thyroid hormone (TH) signaling on the progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD) across 90 patients and experimental models.
Serum levels of TH were measured and correlated with renal function, revealing a strong association between Free Triiodothyronine (FT3) levels and estimated glomerular filtration rate (eGFR).
Experimentally, treatment of patient-derived tubules and PCK rats (an animal model of ADPKD) with Thyroxine (T4) notably inhibited cyst formation and growth, improved renal function, and reduced proteinuria.
The study highlights that TH, particularly T4, may play a protective role in managing ADPKD by modulating cystogenesis through activation of the MAPK cascade via the αvβ3 membrane receptor.
Why is this important?
Understanding the role of thyroid hormones in ADPKD offers a potential therapeutic avenue to slow or even reverse disease progression.
This research not only deepens the insight into the pathophysiological mechanisms of ADPKD but also underscores the potential of TH supplementation as a feasible treatment strategy, especially when administered early in the disease course. The findings advocate for further clinical trials to evaluate the efficacy and safety of thyroid hormone therapy in ADPKD patients.
Join us to end the kidney disease epidemic
Impact of Gut Microbial Metabolite TMAO on Incident Chronic Kidney Disease and Kidney Function Decline
Investigators explored the relationship between the gut microbial metabolite trimethylamine N-oxide (TMAO) and kidney health among 10,564 participants from community-based cohorts in the U.S.
TMAO, generated from dietary components like phosphatidylcholine and carnitine, was linked experimentally to kidney damage. The research utilized mass spectrometry to measure plasma TMAO levels and associated these with changes in kidney function over a median follow-up of 9.4 years.
Elevated TMAO levels significantly correlated with an increased risk of developing chronic kidney disease (CKD) and accelerated annual decline in glomerular filtration rate (eGFR), showcasing a dose-response relationship.
The effects of TMAO on kidney function decline were profound, comparable to known CKD risk factors such as diabetes and hypertension.
Why is this important?
The findings underscore the significant role of diet and dysbiotic gut microbiota in influencing kidney health, particularly through metabolites like TMAO.
These results suggest that interventions aimed at reducing TMAO levels could potentially prevent or slow the progression of CKD, highlighting the need for future clinical trials to explore this therapeutic strategy. Understanding and manipulating gut-derived metabolites offer a promising avenue for addressing kidney disease, highlighting the role of the 5R gut restoration protocol to mitigate CKD risk and progression.
Review article of the month
The Role of Primary Immunodeficiency in Autoimmune Kidney Disease
Primary immunodeficiency (PID) is increasingly recognized not just for its role in predisposing individuals to infections but also for its involvement in autoimmunity, which can lead to severe organ damage including kidney disease.
The complex interplay between PID and autoimmune kidney diseases is mediated through genetic mutations that disrupt immune tolerance, regulatory T-cell function, and other critical immune pathways, resulting in chronic inflammation and tissue damage. Conditions such as lupus nephritis, C3 glomerulopathy, and other kidney-related disorders can manifest in PID patients.
This review article discusses the mechanism behind that and potential role of early and accurate diagnosis of PID, and highlights the need for awareness and advanced genetic and immunological evaluations in clinical settings.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Creating an Integrative Care Plan for Kidney Disease
Kidney disease is a serious condition that requires comprehensive management. An integrative, holistic care plan can be highly effective in managing kidney health and improving the quality of life for patients. Early interventions are crucial in slowing the progression of the disease and preventing complications. Here’s how to create an integrative care plan for kidney disease that emphasizes early interventions for kidney disease, focusing on optimizing individualized care.
Creating an Integrative Care Plan for Kidney Disease
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding Kidney Disease
Chronic Kidney Disease (CKD) is a progressive condition where the kidneys lose their ability to filter blood effectively over time. This can lead to a buildup of waste products in the body, causing various health issues. CKD is a silent epidemic that affects millions worldwide, often escalating to critical conditions before being diagnosed. Early stages often show few or no symptoms, making early detection and intervention vital.
CKD affects more than 1 in 7 adults in the U.S. Diabetes and high blood pressure are responsible for approximately 70% of CKD cases, with most instances of these conditions being linked to lifestyle choices. This means that lifestyle factors play a significant role in the development of CKD. The remaining causes of CKD are largely attributed to genetics, environmental toxins, and autoimmune conditions.
The Importance of Early Interventions
Early detection of CKD is crucial for several reasons, including slowing disease progression, improving quality of life, and reducing the risk of complications. Early interventions can make a significant difference in managing kidney disease. They help in slowing and even reversing disease progression, managing symptoms, and preventing complications. By addressing risk factors and implementing lifestyle changes early on, patients can maintain better kidney function and overall health. This will require creating an integrative care plan for kidney disease.
The Role of Genetics in Kidney Disease Management
Genetics plays a crucial role in personalizing treatment and prevention strategies for kidney disease. This includes understanding genetic predispositions to kidney disease, as well as utilizing nutrigenomics and pharmacogenomics to tailor diet and medication plans.
Nutrigenomics studies how genetic variations affect an individual’s response to nutrients. This field helps in designing personalized dietary plans that can prevent or manage kidney disease more effectively.
Pharmacogenomics examines how genes affect a person’s response to drugs. This knowledge can be used to choose medications and dosages that are most effective for the patient, reducing the risk of adverse reactions and improving treatment outcomes.
Join us to end the kidney disease epidemic
Key Components of a Integrative Care Plan
1. Nutrition
- Dietary Modifications: Nutrition is the mainstay in the integrative approach to kidney disease. High-fiber Mediterranean, DASH, and plant-dominant diets have been found to prevent and slow the progression of kidney disease. In certain kidney disorders, ketogenic and elimination diets can be used. Nutrigenomic data can help create a personalized eating plan that takes genetic predispositions into account.
- Hydration: Proper hydration is essential, but it must be balanced according to the patient’s condition and stage of kidney disease. Low and high water intake have been associated with worse kidney outcomes.
2. Lifestyle Changes
- Exercise: Regular, moderate exercise helps maintain a healthy weight, reduce blood pressure, and improve overall well-being. Activities like walking, swimming, and yoga are excellent choices.
- Stress Management: Techniques such as meditation, mindfulness, and deep-breathing exercises can reduce stress, which is beneficial for kidney health.
3. Herbal and Nutritional Supplements
- Herbal Remedies: Certain herbs like nettle leaf, astragalus, cordyceps, and dandelion root may support kidney health. Always consult with a healthcare provider before starting any herbal supplements.
- Nutritional Supplements: Supplements such as omega-3 fatty acids, vitamin D, and prebiotics, probiotics, and postbiotics can support overall health. Again, it’s important to consult with a healthcare provider.
4. Mind-Body Practices
- Yoga and Tai Chi: These practices not only enhance physical flexibility and strength but also promote mental clarity and stress reduction.
- Acupuncture: This traditional Chinese medicine technique can help alleviate symptoms and improve kidney function.
5. Medical Management
- Regular Monitoring: Frequent check-ups with a healthcare provider to monitor kidney function and overall health. Blood tests, urine tests, and imaging studies are essential tools. In addition, regular assessment of nutritional status, environmental toxin exposure, and gut health are crucial.
- Medications: Prescribed medications to manage symptoms and underlying conditions such as hypertension or diabetes, tailored through pharmacogenomic insights to enhance efficacy and safety. Addressing the interaction between medication and nutrients is also crucial for the patients.
6. Education, coaching, and Support
- Patient Education: Educating patients about their condition, treatment options, and lifestyle changes is crucial for effective self-management.
- Integrative health coaches play an important role in the integrative approach to kidney health, helping patients navigate their healthcare journeys and empowering them to take control of their health.
- Support Groups: Joining support groups can provide emotional support, practical advice, and a sense of community.
Steps to Develop a Integrative Care Plan for Kidney Disease
Assessment:
The practitioner should begin with a thorough medical assessment to understand the patient’s current health status and specific needs. A comprehensive intake form such as this free form can be helpful.
Goal Setting:
Set realistic, achievable goals for managing kidney disease. These should include both short-term and long-term objectives to guide the patient to succeed.
Personalization:
A tailored care plan can be formulated according to the individual's genetics, needs, preferences, and lifestyle. The plan will vary depending on the type of kidney disease.
Implementation:
The plan can then be implemented with regular monitoring and adjustments as needed. Most plans involve coaching the patient for at least six months.
Evaluation:
Continuous evaluation of the effectiveness of the care plan helps make necessary changes to ensure optimal outcomes.
Join us to end the kidney disease epidemic
The bottom line on Creating an Integrative Care Plan for Kidney Disease
Creating an integrative care plan for kidney disease focusing on early interventions involves a comprehensive approach to health. By integrating medical treatments with nutritional support, lifestyle changes, mind-body practices, and patient education, individuals can manage their condition more effectively and improve their quality of life. Early intervention is key, and a well-rounded, holistic care plan can make all the difference in managing kidney disease. Leveraging genetic information, including insights from nutrigenomics and pharmacogenomics, allows for a more personalized and effective care strategy, optimizing outcomes and enhancing the patient's overall well-being.
By staying informed and proactive, we can safeguard our kidney health. Join us!
Understanding the Uremic Dysbiosis Cycle in Chronic Kidney Disease
Chronic kidney disease (CKD) is a progressive condition characterized by the gradual loss of kidney function. While the direct impact on the kidneys is well-documented, the interplay between CKD and gut health is less commonly discussed. One crucial aspect of this relationship is the uremic dysbiosis cycle, a harmful feedback loop that exacerbates both kidney disease and gut imbalance. In this blog, we will delve into the intricacies of the uremic dysbiosis cycle, exploring how it impacts CKD and potential strategies to mitigate its effects.
The Uremic Dysbiosis Cycle
By Majd Isreb, MD, FACP, FASN, IFMCP
What is Uremic Dysbiosis?
Uremic dysbiosis refers to the imbalance of gut microbiota in individuals with CKD or end-stage renal disease (ESRD). In healthy individuals, the gut microbiota plays a crucial role in maintaining overall health, including digestion, immune function, and metabolic processes. However, in CKD, the impaired kidneys lead to the accumulation of uremic toxins in the blood, which can significantly disrupt the gut microbiota's balance.
The Uremic Dysbiosis Cycle Explained
The uremic dysbiosis cycle is a vicious cycle where dysbiosis and CKD perpetuate and exacerbate each other. Here’s a detailed breakdown of the cycle:
1. CKD and Uremic Toxins
In CKD, the kidneys lose their ability to effectively filter waste products from the blood. This results in the accumulation of gut-derived uremic toxins, such as indoxyl sulfate and p-cresyl sulfate. These toxins are harmful not only to the kidneys but also to the gut environment. You can read about these uremic toxins in details in this blog.
Join us to end the kidney disease epidemic
2. Disruption of Gut Microbiota
The presence of uremic toxins disrupts the gut microbiota, leading to dysbiosis. Beneficial bacteria, such as Bifidobacteria and Lactobacilli, decrease in number, while pathogenic bacteria proliferate. This imbalance can affect various bodily functions, including nutrient absorption and immune response.
3. Decreased Production of Short-Chain Fatty Acids (SCFAs)
A healthy gut microbiota produces SCFAs, such as butyrate, which have anti-inflammatory properties and help maintain the integrity of the gut barrier. Dysbiosis leads to a decrease in SCFA production, weakening the gut barrier.
4. Increased Intestinal Permeability
With reduced SCFAs, the gut barrier becomes compromised, resulting in increased intestinal permeability, often referred to as “leaky gut.” This condition allows endotoxins and other harmful substances to pass from the gut into the bloodstream.
5. Systemic Inflammation
The translocation of endotoxins into the bloodstream triggers a systemic inflammatory response. Chronic inflammation is a hallmark of CKD and contributes to its progression by further damaging the kidneys. In addition, elevated gut microbial metabolite such as trimethylamine N-oxide (TMAO) levels significantly correlated with an increased risk of developing chronic kidney disease (CKD) and accelerated decline in glomerular filtration rate (eGFR). In fact, this study showed that effects of TMAO on kidney function decline were so profound, that it was comparable to known CKD risk factors such as diabetes and hypertension.
6. Worsening of CKD
The inflammation and additional kidney damage caused by increased intestinal permeability and endotoxins perpetuate the cycle, worsening CKD. As kidney function continues to decline, the accumulation of uremic toxins increases, further disrupting the gut microbiota.
Breaking the Uremic Dysbiosis Cycle
Addressing the uremic dysbiosis cycle requires a multifaceted approach targeting both gut health and kidney function. A comprehensive gut restoration protocol is vital in this approach. Here are some potential strategies:
Dietary Interventions
Diet plays a crucial role in managing CKD and gut health. Incorporating fiber-rich foods can promote the growth of beneficial gut bacteria and increase SCFA production. Additionally, reducing the intake of foods that contribute to toxin accumulation can alleviate some of the stress on the kidneys.
Probiotics, Prebiotics and Postbiotics
Probiotics, which are live beneficial bacteria, and prebiotics, which are compounds that promote the growth of beneficial bacteria, can help restore gut balance. Probiotic supplements or foods like yogurt and kefir, along with prebiotic-rich foods such as garlic, onions, and bananas, can support a healthier gut microbiota. Postbiotics that provides SCFA can also be beneficial.
Medications and Supplements
Certain medications and supplements may help reduce uremic toxins and inflammation. For instance, oral adsorbents can bind to toxins in the gut and prevent their absorption into the bloodstream. Omega-3 fatty acids and anti-inflammatory supplements may also be beneficial in reducing systemic inflammation.
Regular Monitoring and Medical Support
Regular monitoring of kidney function and gut health is essential for individuals with CKD. Working closely with an Integrative or Functional medicine trained healthcare providers to adjust treatment plans based on the progression of the disease and gut health status can help manage the uremic dysbiosis cycle more effectively.
The Bottom Line on The Uremic Dysbiosis Cycle
The uremic dysbiosis cycle is a complex interplay between gut health and kidney disease, each exacerbating the other in a harmful feedback loop. Understanding this cycle is crucial for developing effective interventions that can break the cycle and improve the quality of life for individuals with CKD. By focusing on dietary changes, probiotics, prebiotics, and medical support, it is possible to mitigate the effects of uremic dysbiosis and support both gut and kidney health.
Breaking the uremic dysbiosis cycle requires a comprehensive approach, but with the right strategies, individuals with CKD can achieve better health outcomes and improve their overall well-being.
By staying informed and proactive, we can safeguard our kidney health. Join us!
Mold Exposure and Kidney Disease: What is the Evidence?
The relationship between mold exposure and kidney disease has garnered significant attention recently. Various studies have linked toxic molds to a range of health issues, including respiratory problems, neurological disorders, and kidney dysfunction. This article explores the connection between mold exposure and kidney disease, shedding light on the underlying mechanisms, preventive measures, and testing methods to safeguard kidney health.
Mold Exposure and Kidney Disease
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding Toxic Mold
Toxic mold refers to certain types of mold that produce harmful “mycotoxins”, which can have severe negative impacts on human health. When these mycotoxins are inhaled or ingested, they can lead to a wide range of health problems. Although some molds are harmless to humans, toxic molds present a significant health risk that must be addressed promptly.
The effects of toxic mold on the human body can vary based on individual susceptibility and the duration and intensity of exposure. Common symptoms of mold-induced health issues include respiratory problems, allergic reactions, and neurological issues. Prolonged exposure to toxic mold can lead to more severe health complications, such as kidney disease and kidney failure.
Mold Exposure and Kidney Disease
Toxic molds, such as Aspergillus, Penicillium, and Stachybotrys chartarum (commonly known as black mold), produce mycotoxins that can adversely affect human health. These mycotoxins can be inhaled or come into direct contact with the skin, entering the bloodstream and impacting various organs, including the kidneys. Notably, Ochratoxin A (OA) and Citrinin, produced by Aspergillus and Penicillium species, are particularly nephrotoxic, meaning they can cause kidney damage.
Join us to end the kidney disease epidemic
Sources of Exposure
Sources of mold exposure are commonly found in environments with excessive moisture. Water damage from leaks, floods, or high humidity can create ideal conditions for mold growth. Poor ventilation in areas like bathrooms, kitchens, and basements can also contribute to mold proliferation.
Additionally, contaminated building materials, such as drywall and insulation, can harbor mold. Mold can also grow on organic materials like wood, paper, and textiles that remain damp.
Due to the presence of organic matter and moisture, occupational settings, particularly those involving agriculture, construction, and waste management, can pose increased risks for mold exposure.
Ochratoxin A has also been found in cereals, coffee, bread, and foods of animal origin. Ochratoxin A is fat-soluble and not readily excreted, so it accumulates in the body. Citrinin was also documented in rice, cereals, fruits, cheese, and dried fruits.
Mechanisms of Kidney Toxicity
Exposure to mycotoxins can lead to several kidney conditions:
- Oxidative Stress: Mycotoxins can induce oxidative stress in kidney cells, leading to cell damage and inflammation. This inflammation can lead to scarring in the kidneys, potentially resulting in chronic kidney disease.
- Toxicity to proximal tubules: Toxic mold exposure can cause acute tubular necrosis, where the kidney tubules are damaged, compromising their function. If not addressed promptly, this condition can lead to acute kidney failure.
- Glomerulonephritis: Deoxynivalenol (DON) is a mycotoxin produced by certain Fusarium species that frequently infect corn, wheat, oats, barley, rice, and other grains in the field or during storage. DON has been documented to cause IgA Nephropathy in mice.
- Other mechanisms of toxicity include nitrosative stress, apoptosis, DNA toxicity and epigenetic modification.
Kidney Disease and Specific Mycotoxins
- Ochratoxin A (OA): This mycotoxin is known for its nephrotoxic, immunosuppressive, carcinogenic, and teratogenic effects. It can cause glomerulosclerosis and has been implicated in conditions like porcine nephropathy and Balkan endemic nephropathy (BEN).
- Citrinin: Produced by various Penicillium species, Citrinin is sometimes found in contaminated rice and can exert significant kidney toxicity. When combined with OA, it may have synergistic effects, increasing the risk of kidney damage.
- Stachybotrys Toxins: Although the connection between Stachybotrys toxins and kidney disease is less clear, some studies have reported mild kidney abnormalities in infants exposed to this mold, including electrolyte disturbances and aminoaciduria. However, these findings are not yet conclusively linked to mycotoxin exposure.
Risk Factors for Mold Exposure and Kidney Disease
Certain groups are more susceptible to mold-related kidney problems:
- Elderly Individuals: Age-related decline in kidney function makes older adults more vulnerable to kidney damage from mold exposure.
- Infants and Young Children: Developing organs and weaker immune systems increase the risk of kidney issues in younger populations.
- Individuals with Compromised Immune Systems: Those undergoing chemotherapy or living with conditions like HIV/AIDS are at higher risk due to their weakened immune defenses.
- People with Pre-existing Kidney Conditions: Individuals already suffering from chronic kidney disease are more susceptible to additional kidney damage from the accumulation of mold and exposure.
Testing for Mold Exposure
Testing for mold exposure is a crucial step in diagnosing and addressing potential health risks, including kidney disease. Several methods can be employed to detect mold exposure:
- Air and Surface Sampling: Environmental testing involves collecting air and surface samples from suspected areas of mold growth. These samples are analyzed in a laboratory to identify the presence and concentration of mold spores and mycotoxins.
- Blood Tests: Blood tests can detect the presence of specific mycotoxins, such as Ochratoxin A and Citrinin. These tests can help confirm exposure and assess the extent of mycotoxin-related damage to the kidneys and other organs.
- Urine Tests: Urine tests are useful for detecting mycotoxins that the body excretes. High levels of mycotoxins in urine may indicate significant exposure and the need for further medical evaluation.
- Urinary Biomarkers: Although numerous biomarkers have been evaluated to assess the harmful effects of various toxicants, β2-microglobulin (β2-MG) and N-acetyl-β-D-glucosaminidase (NAG) are currently the most commonly used biomarkers in environmental toxicology research. However, kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) are also emerging as valuable and informative markers for detecting mycotoxin-induced nephrotoxicity.
Balkan endemic nephropathy
Balkan endemic nephropathy (BEN) is one of the great examples of mycotoxin-induced kidney disease. It is a chronic kidney disease predominantly affecting rural populations in the Balkans. A slow progression to kidney failure follows this. BEN has been strongly linked to mycotoxin exposure, particularly OA. OA has been found in food samples taken from the homes of affected individuals.
Clinical characteristics of BEN include progressive tubular destruction with interstitial and capsular fibrosis. This leads to defects in acidification of the urine, glucose excretion in the urine, increased protein in the urine, and a decrease in GFR. The clearance of p-aminohipurate (PAH), an organic anion, is also decreased in BEN.
These findings can help guide providers suspecting mycotoxin-induced kidney disease. When present, they should prompt further investigation.
Preventing Mold Exposure and Protecting Kidney Health
Preventing exposure to toxic mold is crucial for maintaining kidney health. Here are some effective strategies:
- Address Water Leaks Promptly: Mold thrives in damp environments. Fixing leaks and ensuring proper ventilation can prevent mold growth.
- Reduce Indoor Humidity: Using dehumidifiers to maintain indoor humidity levels below 50% can inhibit mold growth.
- Clean and Dry Damp Areas: Regularly clean areas prone to moisture, such as bathrooms and kitchens, and ensure they are thoroughly dried.
- Proper Ventilation: Ensure good ventilation in living and working spaces to reduce moisture buildup.
- Regular Inspection: Routinely inspect air conditioning systems, ducts, and filters for mold and clean them regularly.
- Avoid contaminated food: Cereals, nuts, spices, dried fruits, apples, and coffee beans may contain mycotoxins, especially when grown under warm and humid conditions.
Detoxifying from mycotoxins requires a comprehensive approach that includes various steps that enhance their release and excretion. Mycotoxins can be stored in tissue, muscle, and fat, so a consistent long-term plan should be devised in collaboration with a Functional Medicine or Integrative Medicine provider. Mycotoxin binders can also be used. This comprehensive blog discusses mycotoxin binders in detail.
Join us to end the kidney disease epidemic
The Bottom Line on Mold Exposure and Kidney Disease
The link between mold exposure and kidney disease underscores the importance of maintaining a mold-free environment to protect kidney health. By understanding the mechanisms through which mycotoxins impact the kidneys, implementing preventive measures, and conducting appropriate testing, individuals can significantly reduce their risk of mold-related kidney issues. If you suspect mold exposure and experience symptoms related to kidney dysfunction, seek medical advice promptly from an Integrative or Functional Medicine provider to ensure early intervention and appropriate treatment.
By staying informed and proactive, we can safeguard our kidney health. Join us!
June Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the June Research and News.
Impact of Propionate and Butyrate on Chronic Kidney Disease Progression
The study, spearheaded by Viviana Corte-Iglesias and her team, is a significant contribution to the field as it delves into the protective roles of short-chain fatty acids (SCFAs), specifically propionate and butyrate, against chronic kidney disease (CKD).
The research evaluated SCFA levels in 54 CKD patients at varying disease stages, revealing that fecal levels of propionate and butyrate decrease as CKD progresses, correlating with worsening clinical markers such as serum creatinine and estimated glomerular filtration rate.
The study also employed genome-wide expression assays to show that propionate and butyrate can significantly reduce inflammatory gene expression in tubular cells under stress.
In addition, a pre-clinical mouse model demonstrated that administering these SCFAs either before or shortly after kidney injury could prevent or slow the progression of renal damage, reduce inflammation, and improve long-term kidney function.
Why is this important?
This research highlights the potential of propionate and butyrate, naturally occurring microbial metabolites, as therapeutic agents in CKD management. By demonstrating their ability to modulate inflammatory responses and improve renal outcomes in a disease model, the study opens new avenues for CKD treatment strategies that leverage the gut-kidney axis.
These findings emphasize the importance of dietary and gut microbial factors in kidney health and support the development of SCFA-based interventions to halt the progression of CKD, offering a promising direction for future Integrative clinical applications.
Permitted Lead Levels in US Drinking Water May Pose Hematologic Risks for Kidney Failure Patients
In a cross-sectional study led by John Danziger, MD, MPhil, the hematologic effects of low-level lead exposure from household water were examined in patients with advanced kidney disease, who are particularly vulnerable to environmental toxins.
The study involved 6404 individuals undergoing dialysis. It revealed that those exposed to higher levels of lead in drinking water required increased dosages of erythropoiesis-stimulating agents (ESAs) and had lower hemoglobin concentrations.
The study also looked at 2648 patients with advanced kidney disease who are not on dialysis. It found that these patients are also more likely to have lower hemoglobin concentrations when exposed to allowable higher levels of lead in drinking water. This was especially more pronounced when there is also iron deficiency.
The analysis identified a clear association between higher household water lead levels and increased hematologic toxicity, as measured by ESA dosing and hemoglobin levels.
This suggests that even the low levels of lead commonly found in US household water can have significant adverse health impacts on individuals with kidney disease.
Why is this important?
This study highlights the critical health risks posed by seemingly safe levels of lead in drinking water, particularly for individuals with chronic kidney disease.
These findings challenge the adequacy of current water safety standards and emphasize the need for stricter regulations and better protective measures for vulnerable populations.
By demonstrating the tangible effects of environmental lead exposure on a susceptible group, the research calls for immediate public health actions to mitigate lead contamination in drinking water and protect public health.
Therefore, kidney patients should strive to drink only filtered water using trusted filtration systems.
Impact of Preterm Birth on Glomerular Disease Outcomes Across Ages
Jaya Isaac and colleagues conducted an analysis within the Cure Glomerulonephropy (CureGN) cohort to evaluate the long-term effects of preterm birth (<37 weeks' gestation) on glomerular disease outcomes in both children and adults.
The study incorporated 2,205 term and 235 preterm participants, revealing significant findings regarding disease progression and genetic predispositions.
Notably, preterm individuals showed a higher prevalence of Focal Segmental Glomerulosclerosis (FSGS) and APOL1 high-risk genotypes compared to their term counterparts.
Furthermore, the preterm group exhibited quicker progression to significant kidney function decline or kidney failure, and they were less likely to achieve complete remission from proteinuria.
Why is this important?
This study underscores the long-term renal risks associated with preterm birth, highlighting a critical need for targeted monitoring and early intervention strategies for those born preterm.
By establishing a link between preterm birth and more aggressive forms of glomerular diseases, the research signals the necessity for enhanced clinical vigilance and potentially tailored Integrative treatment approaches in this vulnerable population.
Join us to end the kidney disease epidemic
Link Between Physical Activity Intensity, Genetic Predisposition, and Kidney Stone Disease Risk
Yashu Liu and colleagues conducted a comprehensive study within the UK Biobank cohort to evaluate the impact of physical activity intensity on kidney stone disease (KSD), taking into account genetic susceptibility.
This prospective study involved 80,473 participants. It assessed total physical activity (TPA), moderate-to-vigorous intensity physical activity (MVPA), and light-intensity physical activity (LPA) through accelerometers.
A polygenic risk score (PRS) was also developed to gauge genetic risk for KSD.
Over an average follow-up of 6.19 years, the study found that higher quartiles of physical activity across all intensity levels were associated with significantly lower risks of developing kidney stone disease.
Participants in the highest activity quartiles had about a 34- 50% reduced risk compared to those in the lowest activity quartiles, even when accounting for genetic predisposition to KSD.
Why is this important?
This research provides robust evidence supporting the protective effects of physical activity against the development of kidney stones, irrespective of genetic risk.
The findings emphasize the importance of lifestyle modifications as a preventive strategy against kidney stones, potentially influencing public health guidelines and clinical practices regarding KSD management and prevention.
This study also highlights the need for further investigation into the mechanisms by which physical activity mitigates the risk of kidney stones, thereby enriching our understanding and potentially guiding more targeted interventions.
Review article of the month
Vascular and valvular calcifications in chronic kidney disease
Vascular and valvular calcifications contribute to cardiovascular complications and mortality in individuals with chronic kidney disease (CKD). Effective treatments to reduce calcification are ambiguous.
This review explores the underlying pathophysiological mechanisms behind vascular and valvular calcifications, examines the actions of various treatment strategies, and presents recent findings from interventional studies.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Remarkable Benefits of L-Arginine for Kidney Health: Lowering Hypertension and More
L-Arginine, an essential amino acid, plays a pivotal role in various bodily functions and has been increasingly recognized for its significant impact on kidney health. This amino acid is not only crucial for protein synthesis but also offers several benefits for the kidneys, including lowering hypertension, enhancing kidney function, and reducing the risk of kidney disease. In this blog, we will explore three key benefits of L-arginine for kidney health, emphasizing its role in managing hypertension, a major concern for kidney disease patients.
By Majd Isreb, MD, FACP, FASN, IFMCP
L-Arginine Lowers Hypertension: A Gateway to Kidney Health
Hypertension, or high blood pressure (BP), is a leading risk factor for chronic kidney disease. The kidneys contain an intricate network of blood vessels that can be damaged by high blood pressure over time. L-arginine’s role in lowering hypertension is well-documented and is chiefly attributed to its ability to increase nitric oxide production in the body. Nitric oxide is a crucial compound that helps blood vessels relax and dilate, promoting better blood flow and subsequently reducing blood pressure.
Several studies have demonstrated that L-arginine supplements can effectively lower blood pressure in people with mild to moderate hypertension. For instance, This meta-analysis demonstrated that compared with placebo, L-arginine significantly lowered systolic BP by 5.39 mm Hg and diastolic BP by 2.66 mm Hg. This reduction in blood pressure is particularly beneficial for kidney health as it eases the kidney's workload and prevents damage to the renal arteries.
On the other hand, the kidney is a major site of arginine synthesis, where citrulline is converted to arginine by the enzymes argininosuccinate synthase and lyase. The loss of kidney function and mass that occurs in chronic kidney disease (CKD) can lead to increased blood pressure. This rise in BP can be prevented by L-citrulline supplementation. This indicates that the reduction in L-arginine metabolism plays an important factor in CKD-associated hypertension.
Join us to end the kidney disease epidemic
Enhancing Kidney Function Through Improved Circulation
L-Arginine’s ability to boost nitric oxide production not only lowers blood pressure but also enhances overall circulation. Improved blood flow ensures the kidneys receive adequate oxygen and nutrients for proper functioning. Healthy blood flow helps the kidneys efficiently filter waste products from the blood and maintain electrolyte balance, which is vital for the entire body’s health.
In animal models of kidney disease, supplementing with L-arginine was found to improve glomerular filtration rate and renal blood flow after 24 hours of urinary tract obstruction. The NO system also mediated this effect.
L-Arginine and Its Protective Role Against Kidney Disease
L-arginine's antioxidative properties protect kidney health. Oxidative stress is a major contributor to kidney disease progression and managing it can help slow its progression. L-arginine’s ability to enhance glutathione synthesis and nitric oxide production helps reduce oxidative stress by improving blood flow and nutrient delivery to the kidneys.
Moreover, studies suggest that L-arginine supplementation can help reduce kidney inflammation, which is often a response to injury or disease. Reducing inflammation can help protect kidney tissues from further damage and maintain their function. This protective role is essential for individuals at risk of chronic kidney disease, as it can help delay the progression of the condition.
L-Arginine is a Two-Edged Sword
L-arginine supplementation has shown mixed results in various kidney conditions. In animal models of glomerulonephritis, it appears harmful, likely due to increased nitric oxide (NO) production driven by higher levels of inducible NO synthase (iNOS). In humans with chronic glomerular diseases, L-arginine supplementation does not alter the progression of renal disease and should be avoided.
However, several studies have documented the benefits of L-arginine in different forms of chronic kidney disease, including after renal ablation, in cases of ureteral obstruction, diabetic nephropathy, and salt-sensitive hypertension.
Notably, L-arginine levels are decreased in preeclampsia, and recent experimental research suggests that supplementation could prevent it in high-risk pregnancies. Finally, exogenous L-arginine has demonstrated protective effects in ischemic acute renal failure.
Dosing of L-Arginine for Kidney Health
When considering incorporating L-arginine into your regimen for its potential benefits on kidney health, it's crucial to understand the appropriate dosing. The suggested dose of L-arginine varies based on individual health needs and conditions. Generally, a common dosage ranges from 2 to 3 grams per day for adults, effectively lowering hypertension and improving blood flow.
The exact dose for kidney disease is not well established, but in general, we recommend a dose of 1-2 g per day. It's important to consult with a healthcare provider before starting L-arginine. They can offer guidance based on your overall health profile and existing medications. This personalized approach ensures not only the efficacy of the treatment but also its safety, preventing potential interactions and complications.
A Word of Caution: Potential Risks of L-Arginine Supplementation
While L-arginine offers numerous health benefits, it is important to know the potential risks and precautions associated with its supplementation. L-arginine can interact with certain medications, including blood pressure drugs, diabetes medications, and blood thinners, potentially enhancing their effects and leading to complications such as an increased risk of bleeding or severe hypotension.
Additionally, people with certain health conditions, such as asthma, allergies, or herpes, should use L-arginine cautiously, as it may exacerbate symptoms.
As mentioned above, it is crucial to pay attention to the indication of L-arginine in kidney disease. L-arginine should be avoided in glomerulonephritis such IgA nephropathy, FSGS, and lupus nephritis. Ultimately, while L-Arginine benefits many, it should be used under the guidance of a healthcare provider to navigate these potential risks effectively.
The Bottom Line About the Benefits of L-Arginine for Kidney Health
In conclusion, L-arginine offers several benefits for kidney health, particularly in lowering hypertension, improving kidney function through enhanced circulation, and providing a protective effect against certain kidney diseases. However, it should be avoided in patients with glomerulonephritis. While L-arginine supplements are generally considered safe, it is always advisable to consult with a healthcare provider before starting any new supplement, especially for individuals with existing health conditions or those on medication.