Understanding the Link Between Visceral Obesity and Kidney Disease: A Deep Dive
Visceral obesity, commonly known as belly fat, isn't just a matter of aesthetics; it's a serious health concern with far-reaching consequences. Among its many adverse effects, one of the lesser-known yet significant correlations is its role in the progression of kidney disease. In this blog, we'll delve into the intricate relationship between visceral obesity and kidney disease, exploring how excess abdominal fat can exacerbate kidney issues and what steps can be taken to mitigate these risks.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is Visceral Obesity?
Visceral obesity refers to fat accumulation around the abdominal organs, particularly in the intra-abdominal cavity. Unlike subcutaneous fat, which lies beneath the skin, visceral fat surrounds vital organs such as the liver, pancreas, and intestines. This type of fat is metabolically active, releasing hormones and inflammatory substances that can wreak havoc on the body's systems.
Join us to end the kidney disease epidemic
The Prevalence of Visceral Obesity
With sedentary lifestyles and poor dietary habits on the rise, visceral obesity has become increasingly prevalent worldwide. Factors such as genetics, diet, physical activity levels, and hormonal imbalances contribute to the accumulation of visceral fat. Alarmingly, even individuals with a normal body mass index (BMI) can harbor excessive visceral fat, putting them at risk for a host of health problems, including kidney disease.
Understanding Kidney Disease
Chronic kidney disease (CKD) is a progressive condition characterized by the gradual loss of kidney function over time. Common causes of CKD include diabetes, hypertension, and autoimmune disorders. As kidney function declines, waste products and fluids accumulate in the body, leading to a range of complications, including cardiovascular disease, anemia, and bone disorders.
The Connection Between Visceral Obesity and Kidney Disease
Research suggests a strong association between visceral obesity and the development and progression of kidney disease. Excess abdominal fat is linked to insulin resistance, hypertension, dyslipidemia, and systemic inflammation, all of which contribute to kidney damage. Additionally, visceral fat releases adipokines and cytokines that promote inflammation and oxidative stress, further compromising kidney function.
Mechanisms of Injury Between Visceral Obesity and Kidney Disease
Visceral obesity contributes to kidney injury through multiple pathways, including:
- Hemodynamic alterations: Visceral fat can increase renal blood flow and glomerular filtration rate, leading to a series of changes that increase sodium and water retention. This will dilate the kidney blood vessels. The ensuing “glomerular hyperfiltration” can cause a progressive decline in kidney function.
- Metabolic dysfunction: Insulin resistance and dyslipidemia associated with visceral obesity can disrupt renal function and promote the development of kidney disease.
- Inflammation and oxidative stress: Adipose tissue-derived inflammatory mediators can directly damage renal cells and exacerbate existing kidney pathology.
The Connection Between Visceral Obesity, Insulin Resistance, and CKD
Research suggests a strong association between visceral obesity, insulin resistance, and the development and progression of CKD. Excess abdominal fat is not only linked to insulin resistance but also to hypertension, dyslipidemia, and systemic inflammation, all of which contribute to kidney damage.
Insulin resistance, a hallmark of visceral obesity, disrupts the body's ability to effectively utilize insulin, leading to elevated blood glucose levels and increased insulin production by the pancreas. This metabolic dysfunction not only predisposes individuals to type 2 diabetes but also directly impacts kidney health.
Insulin resistance has been implicated in the pathogenesis of diabetic nephropathy, a common cause of chronic kidney disease. It is also a risk factor for chronic kidney disease. Furthermore, insulin resistance promotes sodium retention and sympathetic nervous system activation, contributing to hypertension and renal hemodynamic alterations.
Assessing Visceral Obesity
Assessing visceral obesity is key in understanding its impact on health and identifying individuals at risk for associated complications, including kidney disease. While various methods exist for assessing visceral obesity, one commonly utilized measure is the waist-to-hip ratio (WHR). The WHR compares the waist circumference to that of the hips, providing insight into body fat distribution. A higher WHR indicates greater abdominal adiposity and is associated with an increased risk of metabolic and cardiovascular diseases.
For men, a WHR above 0.90 indicates visceral obesity, while for women, a WHR above 0.85 is associated with increased health risks. However, it's important to note that WHR thresholds may vary based on ethnicity and population demographics.
In addition to WHR, other methods such as waist circumference measurement and imaging techniques like computed tomography (CT) or magnetic resonance imaging (MRI) can provide a more comprehensive assessment of visceral fat accumulation.

Mitigating the Risks of Visceral Obesity and Kidney Disease
Addressing visceral obesity is paramount in reducing the risk of kidney disease progression. Lifestyle modifications such as regular exercise, a balanced diet low in processed foods and sugars, and weight management strategies are essential. Additionally, controlling underlying conditions like diabetes and hypertension can help preserve kidney function and prevent further damage.
Join us to end the kidney disease epidemic
The Bottom Line on Visceral Obesity and Kidney Disease
Visceral obesity is more than just a cosmetic concern—it's a significant risk factor for kidney disease progression. Individuals can take proactive steps to mitigate these risks and safeguard their kidney function by understanding the intricate relationship between excess abdominal fat and renal health. Through lifestyle modifications and targeted interventions, we can work towards a healthier future, free from the burdens of visceral obesity and its detrimental effects on kidney health.
May Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the May Research and News.
Kidney Injury Linked to Hair-Straightening Products Containing Glyoxylic Acid
| In a letter to the editor, a case of a 26-year-old Tunisian woman who experienced recurrent acute kidney injury (AKI) following hair-straightening treatments is reported, underscoring potential health risks associated with cosmetic products | Over three instances, coinciding with her salon visits, she developed symptoms including vomiting, diarrhea, fever, and back pain, with laboratory tests indicating elevated plasma creatinine levels.
Notably, the hair-straightening cream used contained 10% glyoxylic acid, suspected of being absorbed through the skin and metabolized into oxalate, leading to calcium oxalate nephropathy. Experimental evidence from mouse models further supported this hypothesis, showing kidney damage and calcium oxalate deposits after topical application of the cream. These findings suggest that glyoxylic acid, a recent substitute for formaldehyde in hair products, may pose significant kidney risks. Why is this important? This case highlights a critical health issue related to widely used cosmetic products, particularly hair-straightening treatments containing glyoxylic acid. The observed link between such products and AKI calls for immediate attention to consumer safety and regulatory oversight. It stresses the need for further investigation into the nephrotoxic potential of cosmetic ingredients and suggests reconsidering the use of glyoxylic acid in consumer products to prevent potential health hazards. Such incidents underscore the broader implications of chemical exposures in everyday products and the importance of rigorous safety evaluations to protect public health. A PDF of this paper is available upon request. For requests, email us at info@inkidney.com |
Gut Microbiota's Role in IgA Nephropathy Through IgA1 Modification
In a groundbreaking study, Gleeson et al. explored how the gut microbiome influences IgA nephropathy, an autoimmune kidney disease.
The research pinpointed that Akkermansia muciniphila, a mucin-degrading bacterium within the gut microbiota, plays a crucial role in the disease's pathogenesis by modifying human immunoglobulin A subclass 1 (IgA1).
This modification involves the deglycosylation of IgA1, which allows it to migrate from the gut to the kidney glomeruli, leading to the formation of damaging immune complexes.
This process was evidenced in both human subjects and mouse models, where mice engineered to express human IgA1 and the human Fc α receptor I developed severe IgA nephropathy upon colonization with A. muciniphila.
The study also highlighted a disrupted correlation between α-defensin 6 and A. muciniphila in patients with IgA nephropathy compared to control subjects, suggesting a protective role of α-defensins against microbiota-induced autoimmunity.
Why is this important?
This research provides critical insights into the link between gut microbiota dysbiosis and autoimmune diseases, specifically IgA nephropathy.
By identifying the molecular interactions between gut bacteria and the immune system, the study opens new avenues for targeted therapeutic strategies that could modify gut microbiota or inhibit specific bacterial actions to prevent or treat autoimmune conditions.
Understanding the role of gut microbiota in immune system modulation not only enhances our comprehension of autoimmune pathogenesis but also underscores the potential of microbiome-focused therapies in clinical settings. This could lead to innovative therapies that manage or prevent diseases by restoring microbial balance, thus improving patient outcomes in autoimmune disorders like IgA nephropathy.
Visceral Adiposity Impacts ADPKD Progression
In a retrospective cohort study, researchers investigated the role of visceral adiposity in the progression of autosomal dominant polycystic kidney disease (ADPKD) among participants of the TEMPO 3:4 tolvaptan trial.
The study included 1053 ADPKD patients identified as high-risk for rapid disease progression. Utilizing deep learning to analyze MRIs, researchers quantified visceral adiposity and examined its association with changes in total kidney volume (TKV) and the efficacy of tolvaptan treatment.
The results revealed that higher levels of visceral adiposity were significantly linked to greater annual increases in TKV, particularly in the highest tertile of adiposity, which showed a notable correlation with rapid kidney growth.
This association was stronger in females than in males. Additionally, the effectiveness of tolvaptan in slowing kidney growth was diminished in patients with higher visceral adiposity.
Intriguingly, while greater visceral adiposity did not globally correlate with the rate of decline in estimated glomerular filtration rate (eGFR), it was associated with a more rapid decline in eGFR among females.
Why is this important?
This study highlights the significant impact of visceral adiposity on the progression of ADPKD and the modulation of treatment efficacy, underscoring the need for personalized treatment approaches that consider body composition.
By demonstrating that visceral fat is more than just a passive storage site but an active endocrine organ influencing disease progression, this research prompts a reevaluation of how ADPKD is managed, particularly in patients with normal BMI who may still be at risk due to high visceral fat levels.
Patients with ADPKD would want to get rid of their Visceral fat as much as possible, and the most effective way to do this is ketogenic metabolic therapy (KMT). Work with an experienced dietitian, nutritionist, or doctor to accomplish this.
Join us to end the kidney disease epidemic
Dietary Potassium Intake and Hyperkalemia Risk in CKD Patients
In a detailed investigation, researchers examined the association between dietary potassium intake from various food sources and hyperkalemia in patients with non-dialysis-dependent chronic kidney disease (CKD).
Conducted in Tokyo with 285 CKD patients, the study utilized a validated diet history questionnaire to estimate dietary potassium intake and assess its impact on serum potassium levels and hyperkalemia risk, defined as serum potassium ≥5.0 mEq/L.
The findings indicated a weak association between total potassium intake and serum potassium levels, but no significant link with hyperkalemia risk was observed.
However, specific food groups such as potatoes, pulses, and green/yellow vegetables positively correlated with serum potassium. Notably, patients consuming high amounts of potassium from potatoes exhibited a markedly increased risk of hyperkalemia compared to those with lower intake levels.
Why is this important?
This research underscores the nuanced role of dietary potassium in managing CKD, particularly highlighting how different food sources of potassium can differently affect the risk of hyperkalemia.
The strong association between potato-based potassium intake and increased hyperkalemia risk suggests that dietary recommendations for CKD patients should not only focus on the amount of potassium intake but also consider its sources.
These insights are crucial for clinicians and dietitians in tailoring dietary plans that minimize hyperkalemia risk while managing the nutritional needs of CKD patients, ultimately improving patient care and outcomes in CKD management.
Review article of the month
Does treatment of metabolic acidosis slow CKD progression?
Metabolic acidosis is a common complication in patients with chronic kidney disease (CKD), becoming more prevalent as kidney function declines.
Nephrologists have long relied on alkali therapy to normalize serum bicarbonate levels and potentially slow the progression of CKD, a practice supported by clinical guidelines based on animal studies and some contentious clinical trials.
This review scrutinizes the evidence behind the routine use of alkali therapy in CKD management. Given the shift in upcoming KDIGO guidelines, which propose downgrading the treatment of metabolic acidosis from a formal recommendation to a practice point, this critical appraisal is particularly pertinent.
The review aims to ascertain whether the current evidence justifies this widespread practice amidst evolving clinical guidelines.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Rethinking Dietary Potassium Intake in CKD: The Emerging Benefits of Plant-Based Diets in Kidney Disease Management
Chronic kidney disease (CKD) affects millions worldwide, imposing significant dietary challenges, particularly regarding potassium intake. Traditionally, CKD patients have been advised to limit potassium due to the risk of hyperkalemia, a condition characterized by elevated potassium levels that can lead to serious heart complications. However, recent research is shifting this perspective, especially concerning plant-based diets. Here, we will focus on dietary potassium intake in CKD.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Traditional View and Its Limitations on Potassium Intake in CKD
Historically, the management of CKD involved stringent dietary restrictions, including limiting the intake of potassium-rich foods like fruits, vegetables, and legumes. This approach was intended to prevent hyperkalemia, which can cause muscle weakness, heart palpitations, and even life-threatening arrhythmias. However, this restriction often resulted in diets that lacked variety and essential nutrients, potentially depriving patients of the benefits of a balanced diet.
Join us to end the kidney disease epidemic
New Insights into Plant-Based Diets and Potassium Intake in CKD
Emerging research suggests that plant-based diets can benefit CKD patients without necessarily increasing the risk of hyperkalemia. Studies indicate that a plant-based diet, which is high in fruits, vegetables, grains, and nuts, can help slow the progression of CKD and improve overall health outcomes. These diets are naturally lower in protein, which helps reduce the kidney's workload, and they provide a rich array of nutrients that support overall health.
Interestingly, despite the high potassium content in many plant foods, recent findings show that plant-based diets do not increase serum potassium levels more than non-plant-based diets in CKD patients. In fact, this study showed that there is a weak association between total potassium intake and serum potassium levels and no significant link with hyperkalemia risk.
The health benefits of plant-based diets, which are often high in potassium, could stem from their ability to create an alkaline environment in the body. This alkalinity may facilitate the movement of potassium into cells, an effect enhanced by insulin stimulation. Furthermore, alkalinity tends to increase potassium excretion by the kidneys.
Additionally, the high fiber content found in plant foods aids in increasing potassium excretion through feces, further balancing potassium levels.
What Are the Benefits of Plant-Based Diets for CKD Patients?
Plant-based diets were also found to prevent kidney disease. The evidence also shows the benefits of plant-based foods in cardiovascular health. A plant-based diet offered a 24% risk reduction in heart disease.
Additionally, the data discussed in this systematic review concluded that plant-based diets are associated with lower blood pressure and overall better health outcomes (namely, cardiovascular system) than animal-based diets.
Higher potassium content in plant-based food may be responsible for these benefits. Increased potassium intake increases the kidney’s ability to excrete sodium, an important driver for higher blood pressure. On the other hand, potassium restrictions have been found to increase sodium retention. However, it is possible that these effects are blunted in advanced kidney disease.
Understanding the nutritional science behind these benefits reveals even more about their effectiveness, and the gut plays an important role here, too.
The Gut-Kidney Connection in Potassium Intake in CKD
The gut also plays an important role in balancing potassium levels in the blood and its excretion by the kidneys. This study, for example, suggests that how our bodies handle potassium from our diet is more complex than previously thought.
Traditionally, it was believed that eating potassium-rich foods leads to increased potassium levels in the blood, promoting its excretion through the kidneys. However, the study found that even when blood potassium levels were the same, rats that ate potassium-rich food had a higher potassium excretion rate than those that received potassium through an infusion.
This indicates that the gut directly senses dietary potassium and signals the kidneys to excrete more, independent of the blood potassium levels. This process does not involve changes in aldosterone, a hormone typically involved in regulating potassium. This suggests a more proactive, or "feedforward," mechanism by the gut in managing potassium levels after eating.
The Right Plant-Based Diet
The popularity of plant-based foods in the food industry has surged, with many grocery stores now offering dedicated sections for these products. However, it's important to note that not all plant-based foods are created equal. Many items in these sections are highly processed and could contain additives unsuitable for those with kidney issues.
In fact, this study showed that an “unhealthy” plant-based diet increased the modestly heightened risk for both CKD progression and all-cause mortality.
A healthier approach to a plant-based diet involves focusing on fresh, minimally processed whole foods and reducing the intake of animal products. An exemplary model of this diet is the Mediterranean diet, renowned for its potential benefits in slowing the progression of kidney disease.
Clinical Studies and Patient Experiences
Clinical evidence supports the safety and efficacy of plant-based diets in managing CKD. For instance, a study involving CKD patients who followed a plant-based diet found no significant increase in potassium levels compared to those on traditional diets. These patients also reported better control over their health and improved quality of life.
Furthermore, personal testimonials from CKD patients who have adopted plant-based diets highlight significant health improvements, including weight loss, better management of symptoms, and reduced dependency on medications.
Join us to end the kidney disease epidemic
The Imperative of Personalizing Dietary Recommendations for Potassium Intake in CKD
The importance of individualizing dietary recommendations in managing CKD cannot be overstated. Each patient's condition presents unique challenges and nutritional needs, making a one-size-fits-all diet approach potentially ineffective or even harmful.
Factors such as the stage of kidney disease, other health issues like diabetes or hypertension, and individual nutritional deficiencies play critical roles in shaping a diet plan. In addition, some medications may lead to potassium retention.
Experienced integrative renal dietitians are key in this process; they help tailor eating plans that manage potassium levels, maintain nutritional balance, and enhance overall health. This personalized approach ensures that dietary management is both effective and sustainable, aiding in the patient's overall well-being and disease management.
The Bottom Line on Dietary Potassium Intake in CKD
The evolving understanding of potassium management in CKD underscores the importance of reevaluating patient dietary guidelines. With new research supporting the benefits of plant-based diets, it's becoming clear that these diets can offer a viable nutritional strategy for managing kidney disease, enhancing patient well-being, and potentially transforming the dietary landscape for CKD patients.
As research continues to unfold, CKD patients are encouraged to consult with healthcare professionals to understand better how to integrate these new insights into their dietary management plans. Discuss these findings with your healthcare provider and read further through links to studies or dietary guidelines provided in this blog.
The Effects of Creatine Supplements on the Kidneys
Creatine supplements are widely popular among athletes and fitness enthusiasts for improving muscle strength and performance. However, there's a growing discussion about the potential health implications of long-term creatine use, particularly concerning kidney health. In this blog, we will explore the effects of creatine supplements on the kidneys, backed by scientific research and expert opinions.
By Majd Isreb, MD, FACP, FASN, IFMCP
Introduction to Creatine and Its Popularity
Creatine is a natural substance found in muscle cells, where it helps produce energy during high-intensity exercise or heavy lifting. In supplement form, creatine is often taken to increase muscle mass, enhance strength, and improve exercise performance. Its popularity is evidenced by its widespread use among athletes, bodybuilders, and regular gym-goers looking to gain a competitive edge or improve their fitness levels.
Effects of Creatine Supplements on the Kidneys
The Role of Creatine in the Body
Before discussing the impacts on kidney health, it’s important to understand how creatine functions within the body. When consumed, creatine increases the stores of phosphocreatine in the muscles, which is used to produce more ATP (adenosine triphosphate), the key energy carrier in cells. This process is crucial during times of stress and high energy demand, such as intense physical activity.
Join us to end the kidney disease epidemic
Creatine Metabolism and Kidney Function
The metabolism of creatine produces creatinine, a waste product that the kidneys filter out of the blood. Typically, creatinine levels in the blood are a marker used by medical professionals to evaluate kidney function. Elevated creatinine levels can indicate that the kidneys are not functioning properly.
Research on Creatine and Kidney Health
Numerous studies have investigated whether taking creatine supplements could lead to kidney damage. For healthy individuals, there is no conclusive evidence that moderate creatine supplementation causes harmful effects on the kidneys. A position statement by the International Society of Sports Nutrition published in the "Journal of the International Society of Sports Nutrition" concluded that creatine supplementation is safe.
A review article looked at the safety of creatine supplementation on kidney health. The review found that these supplements are safe for healthy adults at the recommended loading dose of 20 gm/day for five days followed by a maintenance dose of </= 3 gm/day. The review noted an isolated case report of acute kidney injury with the prolonged intake of high doses of creatine (20 gm/d)
However, creatine supplementation may complicate the care of patients with kidney disease since it will increase serum creatinine making measurement of kidney function less accurate. Therefore, caution is advised for those with pre-existing kidney conditions. Some case reports and smaller studies suggest that individuals with renal disease or reduced kidney function should avoid creatine supplements as it may exacerbate their condition.
Creatine supplements and serum creatinine levels
Creatine supplements are known to increase serum creatinine levels, a common biomarker used to assess kidney function. This increase, however, does not necessarily indicate kidney damage but rather reflects an increase in the production of creatinine, a breakdown product of creatine used by muscles.
A review article noted that while creatine supplementation does elevate serum creatinine, it does not impair kidney function in healthy individuals without pre-existing kidney conditions. This suggests that the creatinine level changes are due to increased muscle creatine content rather than actual renal dysfunction.
Therefore, it's important for both clinicians and patients to understand that creatine supplementation can alter creatinine levels and may affect the interpretation of kidney function tests without necessarily indicating underlying kidney damage.
Practical Tips for Patients Taking Creatine Supplements
It is important for both clinicians and patients to understand that creatine supplementation can increase serum creatinine levels. The following signs can guide the clinician that the elevation in serum creatinine is false and does not warrant any further evaluation:
- The BUN (blood urea nitrogen) is normal. BUN is another waste product that can be elevated in kidney disease. It is not usually accurate, so it is not widely used to estimate kidney function. Patients who are taking creatine supplements will have increased serum creatinine but normal BUN. However, caution should be taken when interpreting this in the face of high protein intake, which may elevate BUN.
- Urinalysis (UA) and urine albumin to creatinine ratio (UACR) are normal. Any abnormalities in UA or UACR may indicate ongoing kidney damage.
- Creatinine clearance is measured by collecting urine for 24 hours. Nephrologists frequently used this measurement to assess kidney function before the development of various equations to estimate GFR. Those taking creatine supplements should have a normal creatinine clearance test.
- Measure Cystatin C levels. Cystatin C is another waste product that is gaining more popularity in estimating GFR. Its level should be normal in patients taking creatine supplements.
- Absence of any other signs of kidney disease, such as electrolyte or acid-base abnormalities.
Join us to end the kidney disease epidemic
Expert Opinions and Recommendations
Medical professionals and nutrition experts generally agree that creatine supplementation is safe. However, they should be used with caution in individuals with known kidney issues. Regular monitoring of kidney function through blood and urine tests is recommended for those who choose to take creatine, particularly if they are using high doses or taking it for extended periods.
Safe Usage Guidelines
For those considering creatine supplements, the following guidelines can help minimize any potential risks:
- Consult a Healthcare Provider: Before starting any supplement regimen, particularly creatine, it’s important to consult with a healthcare provider. This is especially crucial for individuals with pre-existing health conditions or those taking other medications.
- Follow Recommended Doses: Sticking to the recommended dosages can help prevent the potential buildup of creatinine in the blood. The general recommendation for creatine supplementation is 3-5 grams per day after an initial loading phase if one is undertaken.
- Stay Hydrated: Adequate hydration is crucial when taking creatine, as it helps support kidney function and creatinine excretion.
The Bottom Line on Creatine and Kidney Health
Creatine remains one of the most popular and extensively studied supplements in the fitness and sports industry. While it is generally safe for healthy individuals, those with kidney conditions should proceed with caution and under medical supervision. By following proper dosing guidelines and consulting healthcare professionals, most people can safely enjoy the benefits of creatine without compromising their kidney health.
Navigating Sodium Intake in CKD: Essential Tips for Kidney and Cardiovascular Health
Sodium, a vital mineral in our diet, plays a crucial role in maintaining fluid balance and supporting nerve and muscle function. However, striking the right balance in sodium intake is critical for both kidney and cardiovascular health. Recent studies have shown that both excessive and insufficient sodium intake can lead to adverse health outcomes, including cardiovascular mortality. This blog explores the dual role of sodium in blood pressure (BP) control and provides effective strategies for managing sodium intake to support overall health. It focuses on sodium intake in CKD.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Double-Edged Sword of Sodium Intake
Understanding the Impact on Blood Pressure
The effect of sodium balance on blood pressure control is well-documented, making it a key dietary focus for those looking to maintain cardiovascular health. Excess sodium intake leads to a complex response in the body. However, the kidneys' handling of sodium and changes in the blood vessels are the most crucial.
High sodium intake leads to twofold changes in the blood vessels, which increase their tonicity and raise blood pressure:
- It causes increased stiffness of the cells lining the blood vessels.
- It decreases the production of nitric oxide (NO), which dilates the blood vessels. Therefore, high sodium intake leads to blood vessel constriction by lowering the levels of NO.
The Renin-Angiotensin-Aldosterone System
Typically, elevated sodium intake decreases the kidneys' production of a hormone called renin. Renin usually increases the production of angiotensin, which leads to vascular constriction. Angiotensin also stimulates the adrenal glands to produce a hormone called Aldosterone, which usually causes the kidneys to increase the excretion of sodium.
So, the decrease in angiotensin and aldosterone production helps the body get rid of the extra sodium and decrease blood pressure. This is called the renin-angiotensin-aldosterone system (RAAS). This system provides instantaneous feedback that helps the body regulate the sodium levels in the blood and blood pressure.
However, studies have shown that this system has limited capacity. So, at higher levels of sodium intake, aldosterone continues to be produced, which leads to sodium retention and increased blood pressure.
Sodium Intake and Health Outcomes
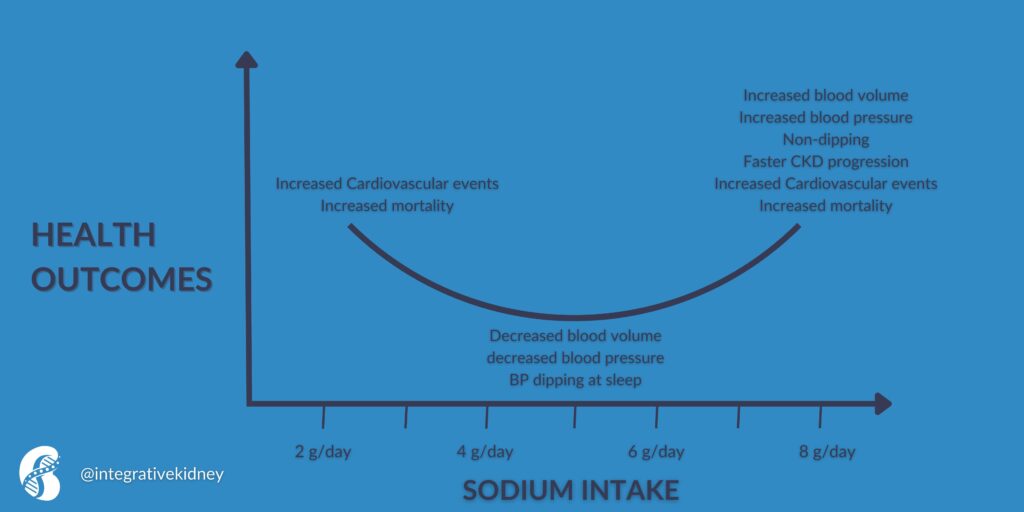
While high sodium intake has been found to increase blood volume and blood pressure. It was also found to decrease the normal dipping pattern of blood pressure during sleep. Elevated blood pressure is well known to increase cardiovascular events and mortality. Moderate restrictions of sodium were found to reverse all these effects.
However, excessive sodium restrictions were also found to increase the risk of cardiovascular events and death. This is believed to be due to the activation of the renin-angiotensin-aldosterone system and the increase in catecholamines (the fight-or-flight hormones).
This effect is summarized in this figure.
Sodium and Kidney Health
The kidneys play a pivotal role in sodium balance, filtering excess sodium into the urine. The renin-angiotensin-aldosterone system plays a major role in this process. In addition, the kidneys have an intricate system that helps them detect excessive sodium in the body and excrete sodium from various tubular segments. This process is crucial for body fluid maintenance and blood pressure regulation.
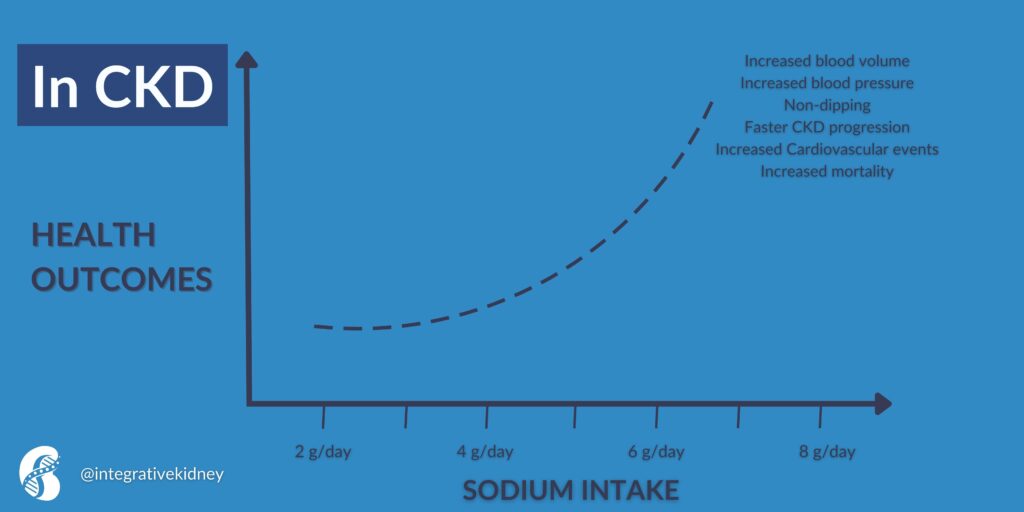
In chronic kidney disease (CKD), the body's usual ability to handle increased sodium is weakened. This is because of the activation of the RAAS. Even though there is more sodium and fluid in the body, the activation of this system causes blood vessels to narrow and the body to hold onto sodium.
Other defense mechanisms, such as the production of NO, which helps blood vessels relax, are also weakened in CKD. This is why consuming a lot of sodium (more than 5 grams per day) can cause a significant increase in both blood volume and blood pressure.
Therefore, in CKD, high dietary sodium intake is associated with increased cardiovascular events and mortality. This is summarized in this figure.
Effects of High Sodium Intake in CKD
High sodium intake in CKD can lead to worse health outcomes. High sodium intake was found to increase the risk of progression in CKD. This is partly due to increasing blood pressure, which is known to increase the progression of CKD. However, high sodium intake was also found to increase sympathetic tone, oxidative stress, inflammation, vascular stiffness, and fibrosis in CKD. Higher sodium intake was also associated with an increase in proteinuria (protein in the urine).
On the other hand, excessive short-term restriction of sodium intake (less than 2 g/day) is found to decrease estimated GFR. It is not clear if this is associated with a long-term impact on kidney disease. Currently, the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines for the management of blood pressure in chronic kidney disease recommends that CKD patients with high blood pressure reduce their salt intake to less than 2 g/d of sodium.
Strategies for Balanced Sodium Intake in CKD
Assessing Your Sodium Needs
The first step in managing sodium intake is understanding your personal sodium needs, which can vary based on age, lifestyle, and health conditions. The general recommendation by health organizations is to limit sodium to less than 2,300 milligrams per day, roughly equivalent to a teaspoon of salt. However, for those with hypertension or kidney issues, even lower levels may be advisable. Consulting with a healthcare provider can help tailor these recommendations to individual needs.
Reading Food Labels Carefully
Packaged and processed foods contribute significantly to sodium intake. Learning to read food labels can be a game-changer in controlling sodium consumption. Look for terms like "low sodium," "reduced sodium," or "no salt added," and be mindful of serving sizes as they relate to sodium content.
Incorporating Natural Sodium Sources
Instead of relying on added salts, incorporating natural sources of sodium can help manage intake levels. Foods like celery, beets, and carrots contain sodium naturally and provide additional nutrients beneficial for health.
Cooking and Seasoning Wisely
Home cooking is a powerful way to control sodium. Use herbs, spices, and salt-free seasoning blends to enhance flavor without adding extra sodium. Experimenting with lemon juice, vinegar, and various spice combinations can bring new life to dishes without compromising health. Salt substitutes can be used and were found to lower blood pressure. These substitutes contain potassium chloride and should be used with caution in patients with advanced CKD. Potassium citrate might be a better option.
The Bottom Line on Sodium Intake in CKD
Sodium intake requires careful consideration, especially for those concerned with kidney and cardiovascular health. By understanding the impacts of sodium, both high and low, and employing strategies to manage intake, individuals can protect their health while enjoying a flavorful diet. Remember, balance is key in maintaining optimal health outcomes related to sodium consumption.
This comprehensive approach to understanding and managing sodium intake in CKD highlights the importance of moderation and provides practical tips for maintaining kidney and cardiovascular health. Remember, individual needs can vary, so it's important to consult with healthcare providers when making significant changes to your diet.
Understanding Drug-Induced Kidney Stones: Causes, Risks, and Prevention Strategies
Kidney stones, medically known as nephrolithiasis, are a common health concern that affects millions of people worldwide. These hard mineral deposits form in the kidneys and can cause severe pain, urinary problems, and even kidney damage if not properly managed. While factors such as diet, hydration, and genetics play significant roles in the development of kidney stones, medications can also contribute to their formation. This article explores the connection between certain medications and kidney stones, providing insights into the prevention and management of drug-induced kidney stones.
By Majd Isreb, MD, FACP, FASN, IFMCP
Drug-Induced Kidney Stones
Types of Kidney Stones
Kidney stones can be classified into several types based on their chemical composition: calcium oxalate, uric acid, struvite, and cystine stones. Each type has its unique causes and risk factors. For example, calcium oxalate stones, the most common type, may result from high oxalate levels in the diet, while uric acid stones are often associated with high protein diets and conditions such as gout.
Prevalence of Kidney Stones
The global prevalence of kidney stones is on the rise, now affecting approximately 1 in 10 individuals during their lifetime. This upward trend is linked to dietary changes, increased body weight, and certain health conditions. Moreover, kidney stones have a notable recurrence rate, with half of the individuals experiencing a recurrence within 10 years, and this rate increases to 75% after 20 years. Furthermore, kidney stones contribute to up to 5% of kidney failure cases that necessitate renal replacement therapy, such as dialysis or transplantation.
Given these statistics, the importance of implementing preventive measures cannot be overstated. A deep understanding of the various types of kidney stones and their increasing prevalence is essential for identifying at-risk individuals and adopting effective strategies to prevent stone formation.
Join us to end the kidney disease epidemic
Drug-Induced Kidney Stones
A less commonly known cause of kidney stones is the long-term use of certain medications. Drug-induced kidney stones account for 1-2% of all kidney stones. These drug-induced kidney stones can form when a drug or its metabolites crystallize in the kidneys, leading to stone formation. This process can be influenced by several risk factors:
- Long-term treatment: Prolonged use of specific medications increases the likelihood of stone formation.
- High doses: Higher doses of these medications can raise the concentration of drugs or their metabolites in the urine, contributing to stone formation.
- Poor solubility in urine: Drugs or their metabolites that are poorly soluble in urine are more likely to form crystals and, subsequently, stones.
- Co-existing patient-dependent risk factors: Several patient-related risk factors have been found to increase the risk for drug-induced kidney stones. These include:
-
- Previous history of kidney stones.
- Family history of kidney stones.
- Factors that cause the urine to stagnate, such as horseshoe kidney, bladder diverticula, duplicated urinary system, or prostate enlargement.
- Recurrent UTIs.
- Abnormal urine electrolytes or acidity.
Recognizing these risk factors is key to preventing drug-induced kidney stones, especially in individuals who are already at risk.
Specific Drug-Induced Kidney Stones
There are two major types of drug-induced kidney stones:
- Drug-containing stones: Here, the medication or its metabolites are excreted in the urine, forming crystals and then stones.
- Drugs cause metabolic changes that lead to kidney stones. In this situation, the medication causes stones by interfering with the balance of calcium, oxalate, phosphate, or uric acid or changing the urine pH.
Several medications have been linked to the formation of kidney stones. Here, we highlight five commonly prescribed drugs and discuss their connection to kidney stones:
1. Acyclovir:
Acyclovir is used to treat herpes infections. It can crystallize in the kidneys, especially when taken in high doses or in patients with reduced kidney function, leading to the formation of stones.
2. Topiramate:
Topiramate is a medication prescribed for epilepsy and migraine prevention. It can increase the pH of urine and decrease urine citrate, promoting the formation of kidney stones, particularly calcium phosphate stones.
3. Ciprofloxacin:
Ciprofloxacin (or Cipro) is an antibiotic used to treat various bacterial infections. When used for extended periods, it can form ciprofloxacin magnesium salt crystals in the urine, increasing the risk of stones.
4. Corticosteroids:
While corticosteroids are used to treat a wide range of conditions, their long-term use can promote the release of calcium from the bones, leading to elevated calcium and phosphate in the urine. They can also increase the level of uric acid in the urine. This contributes to the development of kidney stones in patients receiving long-term corticosteroids.
5. Bactrim:
This antibiotic combination contains sulfamethoxazole, which can be broken down in the body and excreted as N-acetyl sulfamethoxazole, which can crystalize in the urine. In addition, this antibiotic can lead to reduced urine output and changes in urine pH, creating an environment conducive to stone formation.
Bonus: Diuretics
Diuretics like furosemide and triamterene are known to affect mineral balance in the body. Furosemide can increase urine calcium levels, while triamterene and its metabolites can crystalize in the urine, forming kidney stones.
There are many other medications that can increase the risk of kidney stones, especially HIV medication. This reference contains an exhaustive list.
Prevention Strategies for Drug-Induced Kidney Stones
Preventing drug-induced kidney stones involves a multi-faceted approach that takes into consideration the individual's overall health, medication history, and lifestyle factors. Here are several strategies that can significantly reduce the risk:
- Stay Hydrated:
Maintaining adequate hydration is crucial. Drinking plenty of fluids, especially filtered water, helps dilute the urine, reducing the concentration of substances that can lead to stone formation. Aim for at least 2 to 2.5 liters of water per day unless otherwise advised by a healthcare professional.
- Regular Medication Reviews:
Frequent review of your medication regimen with a healthcare provider can help minimize the risk of developing kidney stones. This is particularly important for those on long-term medication, as adjustments in dosage or alternatives may be recommended.
- Dietary Modifications:
Depending on the type of stones (if previously formed) and the medication being taken, dietary changes can be beneficial. For instance, reducing salt intake and avoiding foods high in oxalates (such as spinach, rhubarb, and nuts) may be advised. Consulting with a nutritionist for personalized dietary guidance is recommended.
- Monitor Urine pH:
Some medications affect urine pH, which can promote stone formation. Monitoring and adjusting urine pH through diet or medication under medical supervision can help manage this risk.
- Supplementation and Medications:
In certain cases, supplements or medications may be prescribed to prevent stone formation. For example, potassium citrate is often used to prevent the formation of calcium oxalate, uric acid, and cystine stones by alkalinizing the urine.
- Lifestyle Adjustments:
Maintaining a healthy weight, engaging in regular physical activity, and avoiding smoking can also reduce the risk of kidney stones.
- Patient Education:
Understanding the potential side effects of medications, including the risk of kidney stones, empowers patients to take proactive steps in their health management. Education on recognizing early signs of kidney stones and when to seek medical help is crucial.
By implementing these prevention strategies, individuals can significantly reduce their risk of developing drug-induced kidney stones. It is important to remember that these strategies should complement, not replace, the advice and treatment plans provided by healthcare professionals.
The Bottom Line
The link between medications and kidney stones underscores the importance of careful medication management, especially for individuals at risk of nephrolithiasis. Patients should not discontinue prescribed medications without consulting their healthcare providers, but awareness of potential risks can guide discussions on preventive measures and alternative treatments. Staying hydrated, monitoring medication intake, and regular medical check-ups can help mitigate the risk of drug-induced kidney stones, contributing to better kidney health and overall well-being.
April Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the April Research and News.
Sweetened Beverage Intake and Chronic Kidney Disease Risk in the UK Biobank
In a cohort study utilizing UK Biobank data, researchers examined the link between sweetened beverage consumption and the onset of chronic kidney disease (CKD). Analyzing 127,830 adults without prior CKD, the study assessed the impact of consuming sugar-sweetened beverages, artificially sweetened beverages, and natural juices on kidney health.
The study's objective was to explore how these beverages affect CKD risk and to assess the potential benefits of substituting these with healthier alternatives like natural juices or water.
Over a median follow-up of 10.5 years, 4459 participants developed incident CKD. The findings revealed that consuming more than one serving per day of sugar-sweetened or artificially sweetened beverages was linked to an increased risk of CKD.
Specifically, sugar-sweetened beverages posed a 19% higher risk, while artificially sweetened beverages showed a 10% to 26% increased risk, depending on consumption levels. Interestingly, natural juice intake did not significantly affect CKD risk, indicating no adverse association.
Substitution analyses suggested that replacing one serving per day of sugar-sweetened or artificially sweetened beverages with natural juices or water could reduce CKD risk, highlighting the potential benefits of healthier beverage choices.
Why this is important
These findings underscore the importance of beverage choices in CKD prevention, suggesting that reducing the intake of sugar-sweetened and artificially sweetened beverages may lower CKD risk. By demonstrating the potential health benefits of substituting these beverages with natural juices or water, this study offers practical dietary guidance for individuals aiming to improve kidney health and prevent CKD.
Given the global prevalence of CKD and its association with increased morbidity and mortality, identifying modifiable risk factors like beverage consumption is crucial for public health strategies and individual dietary recommendations.
Gestational Exposure to Maternal Systemic Glucocorticoids Linked to Childhood CKD Risk
In a retrospective cohort study, investigators explored the potential link between prenatal exposure to maternal systemic glucocorticoids (SG) and the development of chronic kidney disease (CKD) in children.
Conducted within Taiwan's largest healthcare delivery system and covering births from 2004 to 2018, the study assessed maternal SG prescriptions during pregnancy as indicators of fetal exposure. The primary focus was on the incidence of CKD in children up to 10 years old, including congenital anomalies of the kidney and urinary tract (CAKUT) and other kidney diseases.
The analysis, involving 23,363 singleton-born children, found that gestational SG exposure was significantly associated with an increased risk of childhood CKD, with an adjusted hazard ratio (aHR) of 1.69.
Notably, the risk was more pronounced for preterm births (less than 37 weeks of gestation), male children, exposure during the second trimester, and a total dose exceeding 24 mg of hydrocortisone equivalent.
Why this is important
These findings highlight a significant association between prenatal exposure to systemic glucocorticoids and an elevated risk of CKD in children, suggesting a potential impact of antenatal SG use on kidney health in offspring.
This supports previous studies that showed similar results. It is, therefore, crucial for healthcare professionals to weigh the risks and benefits of prescribing systemic glucocorticoids during pregnancy. This study emphasizes the need for careful consideration of gestational SG therapy and underscores the importance of monitoring kidney health in children exposed to SG in utero.
Blocking Stomach Acid Does Not Slow The Progression of CKD... Duh!
The VALOR-CKD trial, researchers aimed to explore the effectiveness of veverimer, a novel hydrochloric acid binder, in slowing the progression of chronic kidney disease (CKD) in patients with metabolic acidosis.
This double-blind, placebo-controlled trial, which spanned 320 sites and included 1480 individuals with CKD and metabolic acidosis, found that veverimer did not significantly impact CKD progression compared to the placebo. Notably, the increase in serum bicarbonate levels was minimal, around 1 mEq/L.
Why this is important
This study underscores the complexity of treating metabolic acidosis in CKD and suggests that solely binding stomach acid might not effectively slow CKD progression.
The findings highlight a broader implication for CKD management: the significance of addressing metabolic acidosis through dietary measures, such as an alkaline diet rich in fruits and vegetables, which could offer a safer and potentially more effective strategy.
Such dietary approaches not only avoid the potential deleterious effects of acid binders on the gut microbiome and digestion but also support overall health and kidney function. This trial's outcomes encourage a reevaluation of strategies to combat metabolic acidosis in CKD, emphasizing nutritional interventions over pharmacological ones.
Join us to end the kidney disease epidemic
Serum Uric Acid Levels and Their Impact on Health Outcomes in CKD Patients
In a prospective cohort study, researchers examined the correlation between serum uric acid (SUA) concentrations and mortality risks, including all-cause, cardiovascular disease (CVD), and cancer mortality, in patients with chronic kidney disease (CKD).
The study utilized data from the National Health and Nutrition Examination Survey, which included 6642 patients from 1999 to 2018. It applied weighted restricted cubic spline analyses and multivariate-adjusted Cox proportional hazard models to examine the nonlinearity of these relationships.
Over the follow-up period, totaling 656,885 person-months, the study recorded 2619 all-cause deaths, including 1030 from CVD and 458 from cancer. The results revealed J-shaped non-linear relationships between SUA concentrations and both all-cause and CVD mortality, identifying inflection points at 311.65 μmol/L (5.24 mg/dL) for all-cause mortality and 392.34 μmol/L (6.6 mg/dL) for CVD mortality.
Beyond these inflection points, each increase of 50 μmol/L (0.84 mg/dL) in SUA was linked to an 11.7% and 17.0% higher adjusted hazard ratio for all-cause and CVD mortality, respectively. Conversely, a negative linear correlation was observed with cancer mortality.
Why this is important
This study highlights the significance of monitoring and managing SUA concentrations in CKD patients to potentially enhance long-term health outcomes. Identifying specific inflection points for SUA concentrations in relation to all-cause and CVD mortality offers valuable insights for clinicians in optimizing care strategies for CKD patients.
These findings advocate for further clinical research to determine targeted SUA levels for intervention, emphasizing the importance of personalized medicine in improving survival and quality of life for individuals with CKD.
Review article of the month
Genome-Wide Association Studies Reconstructing Chronic Kidney Disease
Chronic kidney disease (CKD) stands as a global health challenge, ranking as the 16th leading cause of years of life lost worldwide, affecting over 850 million individuals globally, yet suffering from low public awareness and inadequate clinical access.
Despite the known major causes of CKD, such as diabetes mellitus and hypertension, many aspects of its diagnosis remain uncertain. Recent advancements in genetics have highlighted the significant impact of genetic factors on the development and predisposition to CKD.
Through next-generation sequencing and genome-wide association studies (GWAS), researchers are uncovering both rare and common genetic variants linked to CKD, revolutionizing the understanding of the disease. This review article discusses these advances, which deepen insights into CKD mechanisms and open up potential avenues for new therapeutic targets, offering hope for more effective treatments in the future.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Kidney Function Reserve
The concept of Kidney Function Reserve (KFR), analogous to cardiac functional reserve in cardiology, plays a pivotal role in understanding how the kidneys adapt to various physiological and pathological conditions. It is a measure of the kidney's ability to increase its glomerular filtration rate (GFR) - the rate at which the kidney filters blood - in response to certain needs.
By Majd Isreb, MD, FACP, FASN, IFMCP
Essential Functions of the Kidneys: Sustaining Life Beyond Filtration
The kidneys, often undervalued, are essential for overall health and perform their functions quietly until impairment occurs. Every individual has a pair of kidneys positioned on each side of the lower back, comparable in size to a human fist. These remarkable organs process roughly 200 quarts of fluid daily. The primary roles of the kidneys are diverse, covering excretory, endocrine, and metabolic functions.
- Excretory Functions: The kidneys are vital in removing waste products and drugs from the body, maintaining a clean internal environment.
- Endocrine Functions: They are involved in the production of crucial hormones such as erythropoietin, which stimulates red blood cell production, and renin, which plays a key role in blood pressure regulation.
- Metabolic Functions: Additionally, the kidneys are central to activating vitamin D, essential for bone health, and metabolizing certain drugs, thus impacting various physiological processes.
These functions are integral to maintaining blood volume balance, ensuring proper electrolyte and acid-base balance, and contributing to hemostasis, the process that prevents excessive bleeding when an injury occurs. The kidneys' efficient and silent work in these areas is critical for homeostasis, the body's stable internal state.
Limitations of Traditional Kidney Health Markers
Conventional methods for evaluating kidney health, such as serum creatinine levels, urine output, and microscopy, can be misleading. Serum creatinine is affected by factors like muscle mass and diet and may not rise until after significant kidney damage has occurred. In fact, about 50% of kidney function must be lost before a rise in serum creatinine can be detected. Therefore, serum creatinine is a late marker of acute kidney injury.
Changes in urine output can signal early problems but don't directly measure kidney function. Urine microscopy provides insights into kidney damage but not function.
Furthermore, serum creatinine can give false reassurance in critical illnesses like sepsis or hypervolemia due to factors that alter creatinine production or dilute its concentration in the blood. These limitations highlight the need for more direct and reliable markers to assess kidney function.
Understanding Kidney Function Reserve (KFR)
The kidney function reserve (KFR) represents the kidney's capacity to enhance its filtration rate under stress or increased demand, akin to how the heart increases its output during physical exertion. This capacity allows the kidney to maintain overall function and compensate for lost nephrons, delaying the observable effects of declining renal function, such as changes in baseline GFR and serum creatinine levels.
This reserve becomes crucial in both healthy individuals and those with renal impairments, allowing for adaptation to varying conditions such as pregnancy, a solitary kidney, diabetes, nephrotic syndrome, and hypertension.
Measurement and Clinical Significance
KFR is determined by the difference between the peak GFR, achieved through stimuli like protein load, and the baseline GFR. It essentially gauges the kidney's ability to augment its function in response to demand or disease.
For instance, the peak GFR of a person with an intact nephron mass can reach up to 180 ml/min, whereas it may reduce to about 120 ml/min in individuals with a solitary kidney. Despite these conditions, baseline GFR may appear normal, revealing the significant role of RFR in assessing renal health and predicting potential renal disease progression.
Join us to end the kidney disease epidemic
A New Perspective: Kidney Stress Tests
Kidney Stress Tests offer a novel way to assess renal health, akin to cardiac stress tests. They help identify the kidney's functional reserve, which is crucial yet often overlooked in routine care. When kidneys don't respond well to stress, it may indicate early, undetectable damage, which is important for evaluating CKD risk. These tests measure the kidneys' ability to up their filtration rate and function in response to stress, providing essential insights into kidney resilience and health.
An example of kidney stress tests is examining the impact of high protein intake on renal function, which is pivotal in assessing the renal reserve and identifying how kidneys adapt under certain conditions.
Pioneering work by Bosch and others revealed that the glomerular filtration rate (GFR) is not as fixed as once thought; dietary protein can induce hyperfiltration, suggesting that renal performance can vary with diet. These findings challenge the long-held belief that GFR is stable across different individuals and conditions.
High protein intake has also been linked to an increased risk of rapid renal function decline, which supports the use of kidney stress tests to detect and manage potential kidney diseases early. A blunted response to a high-protein diet can detect early kidney damage.
For example, studies of protein-induced hyperfiltration have highlighted that certain groups, including those with CKD stages 1 and 2, diabetics, hypertensive individuals, and patients recovering from acute kidney injuries (AKI), may have an impaired filtration response to protein.
There are other tests to assess various kidney functions that we will discuss in future blogs.
Kidney Function Reserve in Diabetic Patients
Diabetes mellitus poses a unique challenge to RFR, especially in the early stages characterized by hyperfiltration, where high glucose levels stimulate an increased filtration rate. In diabetic individuals, especially those with insulin-dependent diabetes mellitus (IDDM), the early hyperfiltration stage is associated with a subsequent risk of developing diabetic nephropathy (DN).
Studies have shown that the response of the kidneys to an acute protein load in these patients differs markedly from that of healthy individuals, often resulting in a decreased GFR while renal blood flow remains constant. These observations underscore the importance of RFR as a tool for early detection of renal insufficiency before significant impairment or structural injury occurs.
The Bottom Line on Kidney Function Reserve
Assessment of kidney functional reserve is an evolving yet critical aspect of nephrology that offers insights into the kidneys' compensatory mechanisms in response to physiological stress or disease.
It holds promise for early diagnosis, monitoring renal health, and understanding the progression of renal diseases, especially in conditions like diabetes, where early detection can significantly impact patient management and outcomes.
Uric Acid and Kidney Disease - Managing Uric Acid to Protect Against Kidney Disease: A Comprehensive Guide
Uric acid (UA) is often mentioned in discussions about kidney health, gout, and dietary habits. But what exactly is uric acid, and why does it matter? This post delves into the essence of uric acid and its implications on health. We will particularly focus on the association between uric acid and kidney disease (CKD).
By Majd Isreb, MD, FACP, FASN, IFMCP
Uric acid and kidney disease
What is Uric Acid?
Uric acid is a chemical created when the body breaks down substances known as purines, which are found in various foods and drinks. While it's a normal part of the body's processes, excessive uric acid can lead to health issues, including gout, kidney stones, and kidney disease.
Join us to end the kidney disease epidemic
The Connection Between Uric Acid and Chronic Kidney Disease
Research indicates that high uric acid levels can impair endothelial function by reducing nitric oxide levels. This condition, known as hyperuricemia, is linked with vascular diseases like CKD and cardiovascular disease (CVD). Surprisingly, 25% of CKD patients have gout and 60% exhibit hyperuricemia.
Uric Acid's Role in the Rising Incidence of Chronic Kidney Disease
The intriguing relationship between uric acid and CKD has sparked considerable scientific inquiry. According to this review and meta-analysis of eighteen CKD incidence studies, higher uric acid levels were associated with increased CKD incidence. Another meta-analysis found that serum uric acid levels are an independent risk factor for CKD in middle-aged patients. This association was true in different subgroups, including diabetics and patients with elevated lipid levels.
How Uric Acid Affects Kidney Disease Progression
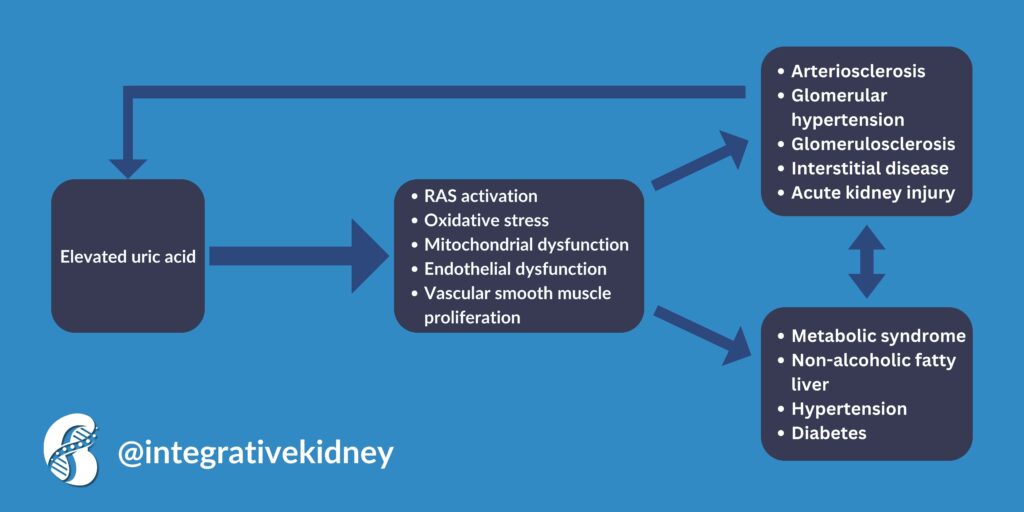
The relationship between uric acid levels and the progression of CKD is complex and still under extensive study. Notably, uric acid concentration is an independent risk factor for kidney failure in early-stage CKD and exhibits a J-shaped relationship with mortality rates in CKD patients.
The cells that line blood vessels are called endothelial cells. Endothelial cells tend to absorb serum uric acid, leading to a decrease in nitric oxide (NO) levels. This occurs as UA inhibits NO production and speeds up its breakdown. Additionally, xanthine oxidase (XO) found in the cytosol and plasma produces superoxide, further reducing NO levels. Consequently, hyperuricemia is linked to compromised endothelial function, which can lead to CKD.
This same mechanism can also lead to oxidative stress, which causes DNA damage, lipid and protein oxidation, cellular inflammation, and death. In fact, uric acid was found to trigger the activation of the immune system and modify the properties of kidney cells, including tubular epithelial cells, endothelial cells, and vascular smooth muscle cells, pushing them toward a state that promotes inflammation and fibrosis.
In short, high uric acid levels were associated with a faster decline in kidney function and rapid progression to kidney failure. The relationship between elevated uric acid and kidney disease is more complex though. We summarized it in the following infographic.
Chronic Kidney Disease and Uric acid
On the other hand, chronic kidney disease by itself can lead to elevated uric acid levels. There are two major causes for elevated uric acid levels in CKD:
- Decreased Renal Excretion: In CKD, the kidneys' ability to filter and excrete waste products is compromised. Because uric acid is primarily eliminated through the kidneys, impaired kidney function can lead to its accumulation in the blood.
- Medication-induced Hyperuricemia: Certain medications, including diuretics (commonly used to treat hypertension and swelling in CKD patients), can reduce the kidneys' ability to remove uric acid, leading to higher serum levels.
Uric Acid and Hypertension
Elevated serum uric acid levels have been implicated in the pathogenesis and evolution of hypertension. This relationship has been observed in both animal and human studies and is recognized as early as childhood and adolescence. Furthermore, treating hyperuricemia with a medication such as allopurinol was found to decrease both systolic and diastolic blood pressure in this meta-analysis.
Hyperuricemia is believed to increase blood pressure through several mechanisms. It was found that it increases renin expression and, therefore, activates the renin-angiotensin system. As mentioned above, hyperuricemia also inhibits endothelial nitric oxide, which can lead to blood vessel constriction. Elevated serum uric acid levels can also lead to salt-sensitive hypertension.
Studies on Uric Acid-Lowering Therapy in CKD
There's evidence suggesting that uric acid-lowering therapy (ULT) could slow the progression of CKD. However, the effects vary with different ULT medications, and not all have shown beneficial outcomes in improving kidney function. For example, topiroxostat and febuxostat were found to slow the progression of CKD, while allopurinol and pegloticase were not. The effectiveness of ULT, including medications like topiroxostat and febuxostat, may vary, particularly among subgroups of CKD patients. So, the medication used to lower uric acid levels matters.
Fructose and uric acid
Fructose consumption plays a significant role in the metabolism of uric acid, establishing a notable link between dietary habits and uric acid levels. When fructose is ingested, it's metabolized in the liver, where one of the byproducts is uric acid. This process can lead to increased levels of uric acid in the bloodstream, contributing to hyperuricemia.
Unlike glucose, which is utilized by cells throughout the body, fructose metabolism is more likely to spike uric acid production. This relationship underscores the impact of diet, particularly high-fructose foods and beverages like sodas and certain fruit juices, on uric acid levels and associated health conditions such as gout and kidney disease.
Understanding this connection is crucial for managing and preventing these conditions through dietary choices.
Natural Ways to Lower Uric Acid Levels
Managing uric acid levels can also be approached through dietary adjustments and lifestyle changes:
- Limiting Purine-rich Foods: Since purines can raise uric acid levels, reducing the intake of foods high in purines, such as certain meats, seafood, and sugary beverages, can be beneficial.
- Incorporating Low Purine Foods: Focusing on a diet that includes low-purine foods like fruits, vegetables, and low-fat dairy products can help manage uric acid levels.
- Avoid Certain Medications: Some medications, such as diuretics and aspirin, can increase uric acid levels. Before making any changes, consult with a healthcare provider.
- Maintaining a Moderate Weight: Obesity, especially visceral obesity, is linked to higher uric acid levels.
- Lifestyle Adjustments: Reducing alcohol intake, increasing fiber and vitamin C consumption, and eating cherries or tart cherry juice are among the suggested strategies to lower uric acid levels.
The Bottom Line
Elevated uric acid levels can lead to health issues such as gout, kidney stones, hypertension, and CKD incidence and progression. However, uric acid levels can be controlled and associated health risks mitigated through a combination of dietary management, lifestyle changes, and, where necessary, appropriate medical treatment.
Unraveling the Mysteries of Nutrigenomics and Kidney Health: How Your Diet Shapes Your Genetic Destiny
In recent years, the field of nutrigenomics has emerged as a powerful tool in understanding how our dietary choices interact with our genetic makeup to influence our health. This exciting discipline explores the intricate relationship between nutrition and genetics, shedding light on how specific nutrients can modulate gene expression and impact various physiological processes within the body. In this blog, we will discuss nutrigenomics and kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Nutrigenomics and kidney health
Nutrigenomics is the study of how nutrients and other dietary components interact with our genes, influencing our individual responses to food and affecting our susceptibility to certain diseases. At its core, nutrigenomics seeks to unravel the complex interplay between diet and genetics, offering insights into how our unique genetic profiles can shape our nutritional requirements and dietary preferences.
The Role of Genetics in Dietary Responses
Our genetic makeup significantly determines how our bodies respond to different nutrients and dietary patterns. Genetic variations can influence enzyme activity, nutrient metabolism, and nutrient absorption, ultimately impacting our nutritional needs and dietary tolerances. By understanding these genetic predispositions, individuals can make more informed dietary choices tailored to their specific genetic profiles.
Gene-Nutrient Interactions
One of the key concepts in nutrigenomics is gene-nutrient interactions, where specific dietary components interact with our genes to modulate gene expression and influence physiological processes. For example, certain nutrients may activate or suppress genes involved in inflammation, oxidative stress, or metabolic regulation, thereby affecting our risk of developing chronic diseases such as obesity, cardiovascular disease, diabetes, kidney disease, and cancer.
Personalized Nutrition Recommendations
Advancements in genetic testing and bioinformatics have paved the way for personalized nutrition recommendations based on an individual's genetic profile. By analyzing genetic data, healthcare professionals can identify genetic variations that may impact nutrient metabolism, dietary requirements, and disease susceptibility, allowing for tailored dietary interventions aimed at optimizing health outcomes and preventing disease.
Nutrigenomics and Chronic Disease Prevention
Nutrigenomics holds immense promise in the prevention and management of chronic diseases. It provides insights into how dietary factors interact with our genes to influence disease risk. By adopting personalized nutrition strategies informed by nutrigenomics, individuals can mitigate genetic predispositions to certain diseases and optimize their health through targeted dietary interventions.
Practical Applications of Nutrigenomics
Incorporating nutrigenomics principles into everyday dietary habits can be empowering for individuals seeking to improve their health and well-being. This may involve choosing nutrient-dense foods rich in bioactive compounds, diversifying dietary patterns to maximize nutritional diversity, and seeking guidance from healthcare professionals trained in personalized nutrition.
Nutrigenomics and Kidney Health
Nutrigenomics offers valuable insights into the intricate relationship between diet and genetics, significantly impacting kidney health and the prevention of kidney diseases. The kidneys, vital for filtering toxins and maintaining fluid balance, are directly influenced by certain dietary factors, which can either exacerbate or mitigate the risk of conditions like chronic kidney disease (CKD) or kidney stones. Understanding genetic predispositions to kidney diseases is crucial; nutrigenomics research aims to identify these variations, guiding the development of personalized dietary strategies to mitigate disease risk.
Nutritional elements such as sodium, potassium, phosphorus, and protein intake have direct effects on kidney function and disease progression. Individuals with CKD often require dietary adjustments to manage electrolyte imbalances and lessen kidney burden.
However, it has been customary to create “blanket diets” for kidney patients. But not all patients are the same. Nutrigenomics can inform tailored dietary recommendations based on an individual's genetic profile, optimizing nutrient intake while minimizing the risk of worsening kidney disease.
Nutrigenomics can also guide the need for supplementing certain trace elements that are beneficial for kidney health. These include zinc, selenium, and CoQ10 for example. Vitamin D3 is also a prime example of nutrigenomics. It exerts its influence through its active form, 1α,25-dihydroxyvitamin D3, which binds tightly to the vitamin D receptor, affecting the epigenome and transcriptome across thousands of locations within the human genome.
Additionally, targeting inflammation and oxidative stress, common features of kidney diseases, is essential. Dietary components like antioxidants and anti-inflammatory compounds found in fruits, vegetables, and omega-3 fatty acids can help alleviate kidney inflammation and oxidative stress. Nutrigenomics delves into genetic factors affecting responses to such interventions, enabling personalized dietary recommendations to support kidney health.
Furthermore, nutrigenomics holds promise in managing metabolic disorders like diabetes and hypertension, leading causes of kidney disease. By understanding how genetic variations impact responses to dietary interventions for these conditions, personalized nutrition recommendations can optimize blood sugar control, blood pressure management, and lipid metabolism, consequently reducing the risk of kidney complications.
Overall, incorporating nutrigenomics principles into personalized nutrition strategies stands to revolutionize kidney disease management, offering hope for improved outcomes and enhanced quality of life for individuals affected by kidney disorders.
Join us to end the kidney disease epidemic
Nutrigenomics and kidney stones
Kidney stones present a significant health challenge. While rare genetic variants have been identified, they account for only a fraction of cases, prompting exploration into broader influences such as environmental exposures and dietary habits.
Nutrigenomics, investigating how diet interacts with our genes, offers promise in understanding the genetic underpinnings of kidney stone formation, while epigenetics, studying changes in gene expression without altering DNA, sheds light on how environmental factors shape risk.
Certain nutrients have been found to affect urinary calcium, oxalate, and citrate via epigenetic and nutrigenomic mechanisms. Acetic acid (vinegar), for example, decreases urinary calcium and oxalate and increases urinary citrate by gene silencing. While fructose consumption was found to increase the risk of kidney stones.
The bottom line on nutrigenomics and kidney health
Nutrigenomics represents a paradigm shift in our understanding of how diet influences genetic expression and shapes our genetic destiny. By embracing personalized nutrition recommendations informed by nutrigenomics, individuals can unlock the potential to optimize their health and mitigate disease risk through tailored dietary interventions. As research in this field continues to advance, the future of personalized nutrition holds tremendous promise in revolutionizing the way we approach kidney health and disease.
March Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the March Research and News.
Reduced Sodium and DASH Diet Impact on Kidney Function: Insights from the DASH-Sodium Trial
In the DASH-Sodium trial, researchers explored the impact of dietary sodium reduction combined with the Dietary Approaches to Stop Hypertension (DASH) diet on kidney function, specifically the estimated glomerular filtration rate (eGFR) measured by cystatin C.
The controlled feeding study involved adults with elevated or stage 1 hypertension who were randomized to either the DASH diet or a control diet, subsequently consuming varying levels of sodium over three 30-day periods.
The study found that while the DASH diet alone did not significantly affect the eGFR, combining it with a low-sodium intake notably decreased the eGFR.
Specifically, transitioning from a high to a low sodium intake resulted in a significant reduction in eGFR. When adjusted for diastolic blood pressure and urinary potassium excretion, the impact of the low sodium-DASH diet on eGFR was somewhat attenuated.
Why is this important?
This study sheds light on the nuanced effects of diet on kidney function, illustrating that a low-sodium version of the DASH diet can lead to a reduction in eGFR over four weeks.
Understanding these dynamics is crucial for developing dietary recommendations, particularly for individuals at risk of hypertension or chronic kidney disease (CKD).
The findings suggest potential implications for the dietary management of kidney health, emphasizing the need for further research to explore the long-term effects of such dietary interventions on kidney function and the progression to CKD.
Join us to end the kidney disease epidemic
Ionized and Total Magnesium Levels in Chronic Kidney Disease: Outcomes and Associated Factors
In this study, investigators examined the relationship between ionized (iMg) and total magnesium (tMg) levels and their impact on major adverse cardiovascular events (MACE) and mortality in patients with chronic kidney disease (CKD).
The study utilized data from the CKD-REIN cohort, focusing on patients with an eGFR of less than 60 mL/min/1.73m², to explore how these magnesium levels correlate with cardiovascular outcomes and mortality before kidney replacement therapy (KRT).
The analysis revealed a strong correlation between serum levels of iMg and tMg, which were both independently associated with eGFR. Interestingly, while the serum tMg levels were linked to an increased risk of MACE and mortality, especially in the highest and lowest tertiles of tMg concentration, the ionized magnesium levels did not show a significant association with these outcomes.
Patients in the highest tertile of serum tMg faced a greater likelihood of experiencing MACE and mortality compared to those in the middle tertile.
Why is this important?
This study underscores the importance of monitoring magnesium levels in CKD patients, given the potential association of total serum magnesium with major cardiovascular events and mortality.
The findings highlight the need for further research to understand the implications of magnesium homeostasis in CKD and its potential as a biomarker or therapeutic target, especially considering the varied results between total and ionized magnesium.
However, the major problem with this study is that it isolated one electrolyte to see its effects on renal and cardiac outcomes. Magnesium in the body does not work alone, and for this study to be really meaningful, it should include levels of other electrolytes and nutrients that magnesium interacts with.
Plasma Levels of Polyunsaturated Fatty Acids and Adverse Kidney Outcomes
In a comprehensive study, researchers examined the association between plasma polyunsaturated fatty acids (PUFA) levels and kidney outcomes in two distinct cohorts from the UK Biobank Study.
Cohort 1 comprised 78,950 participants without chronic kidney disease (CKD), while Cohort 2 included 7,233 participants with CKD.
The study aimed to investigate the relationship between various plasma PUFA components and the risk of incident CKD in the first cohort and the risk of progressing to kidney failure requiring replacement therapy (KFRT) in the second.
The findings indicated that individuals in Cohort 1 with higher plasma PUFA levels were less likely to develop CKD over a median follow-up of 11.9 years. Specifically, the higher quartiles of PUFA showed a significant inverse association with incident CKD, suggesting a protective effect.
In Cohort 2, although total PUFA levels were not linked to the risk of KFRT, higher levels of docosahexaenoic acid (DHA), a specific omega-3 fatty acid, were associated with a reduced risk of progressing to KFRT.
Why is this important?
This study highlights the potential role of polyunsaturated fatty acids in kidney health, showing that higher plasma levels of PUFA are associated with a reduced risk of developing CKD in individuals without pre-existing kidney disease.
Furthermore, for patients with CKD, elevated levels of the omega-3 fatty acid DHA were linked to a lower risk of requiring kidney failure replacement therapy.
These findings suggest that monitoring and potentially modifying plasma PUFA levels could be an important strategy in preventing CKD progression and improving outcomes for individuals with existing kidney disease.
The study provides valuable insights that could inform dietary recommendations and therapeutic interventions aimed at enhancing kidney health and preventing adverse kidney outcomes.
Fructose and Kidney Health: Insights and Implications
In this perspective, published ahead of print in the journal CJASN, researchers discussed the role of fructose in kidney health.
Fructose, commonly found in fruits, honey, and especially in sugar-sweetened beverages like soft drinks, has seen a marked increase in consumption over recent decades. This rise is paralleled by the increased use of high fructose corn syrup, primarily in sodas.
Beyond dietary sources, the body can endogenously produce fructose, especially in conditions such as diabetes, high carbohydrate diets, or excessive alcohol intake.
Both experimental and epidemiological evidence underscores the role of high fructose intake in chronic kidney disease (CKD) progression.
Animal studies have shown that a high-fructose diet leads to significant kidney damage, characterized by tubulointerstitial lesions, fibrosis, increased epithelial expression of vimentin, and heightened immune cell infiltration.
Additionally, fructose exposure has been linked to the release of inflammatory cytokines from cultured proximal tubular cells, correlating with clinical data that associate fructose consumption with an increased incidence of CKD.
Furthermore, endogenous fructose metabolism, particularly in the proximal tubule cells of the kidneys, plays a crucial role in progressive kidney damage.
This metabolic pathway is activated under diabetic conditions and other stressors like renal hypoxia or high salt intake, leading to inflammation, oxidative stress, and subsequent kidney injury.
This is evidenced by animal models where diabetic mice deficient in fructokinase, an enzyme critical in fructose metabolism, exhibit significantly less renal damage compared to their wild-type counterparts.
The pathological implications of fructose metabolism extend to acute kidney injury scenarios, such as ischemic AKI (iAKI), where fructokinase inhibition has shown protective effects against renal injury in animal studies.
This has paved the way for potential therapeutic interventions, such as the use of fructokinase inhibitors like luteolin and osthole, which have shown promising results in mitigating kidney damage in various models.
Why is this important?
The link between fructose intake and metabolism and kidney health is a critical area of research, especially considering the rising prevalence of CKD globally. Understanding how fructose contributes to kidney damage not only illuminates a potential dietary risk factor but also opens new avenues for therapeutic strategies targeting fructokinase inhibition.
Such interventions could offer novel approaches to prevent or mitigate kidney damage, particularly in CKD and iAKI contexts, underscoring the importance of dietary interventions and novel targets in preserving kidney health and preventing disease progression.
Further research in this domain could significantly impact public health strategies and clinical practices, emphasizing the importance of dietary choices and potential new treatments in kidney disease management.
The perspective article can be downloaded for free via this link.
Review article of the month
Plant-derived compounds for treating autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD), the prevailing monogenic hereditary kidney disorder, stands as the fourth primary contributor to end-stage kidney disease on a global scale. Notably, recent advancements have emerged in slowing ADPKD advancement through diverse chemical medications like tolvaptan, rapamycin, and somatostatin.
However, a multitude of plant-derived compounds are found to have the potential to attenuate ADPKD progression. This review article discusses these compounds.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
World Kidney Day 2024: Kidney Health for All
Every year, World Kidney Day serves as a crucial reminder of the importance of our kidneys and the significance of maintaining their health. In 2024, the theme "Kidney Health for All: Advancing equitable access to care and optimal medication practice" underscores the critical need for universal access to kidney health services and medication practices that ensure the best outcomes for everyone. Additionally, this year's focus highlights the importance of early detection of kidney disease and the implementation of lifestyle modifications to promote kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
World Kidney Day 2024: Kidney Health for All
Equitable Access to Care
Access to healthcare is a fundamental human right, yet disparities persist globally, particularly concerning kidney health. Many individuals, especially in low- and middle-income countries, face barriers to accessing essential kidney health services, including screening, diagnosis, treatment, and transplantation. Socioeconomic factors, geographical location, and inadequate healthcare infrastructure contribute to these disparities.
Addressing these challenges requires a concerted effort from governments, healthcare providers, policymakers, and communities. Investments in healthcare infrastructure, training healthcare professionals, and implementing policies that prioritize kidney health can help bridge the gap in access to care. Additionally, promoting awareness and education about kidney health in underserved populations can empower individuals to take proactive measures to protect their kidneys.
Join us to end the kidney disease epidemic
Optimal Medication Practice
Effective medication management is critical in the treatment and management of kidney diseases. However, improper medication practices, including misuse, overuse, or underuse of medications, can exacerbate kidney damage and lead to adverse outcomes. Furthermore, certain medications can be nephrotoxic, meaning they can cause harm to the kidneys if not used appropriately.
Optimizing medication practices involves a comprehensive approach that includes medication reconciliation, monitoring for drug interactions, dose adjustments based on renal function, and patient education on medication adherence. Healthcare providers play a crucial role in ensuring that medications are prescribed judiciously and tailored to individual patients' needs, taking into account their kidney function and other co-morbidities.
Several new medications have been found to slow down the progression of kidney diseases and diminish the protein in the urine. Yet, many patients are not able to access these medications due to their exorbitant costs and barriers placed by various health insurance companies in the United States.
Early Detection and Prevention
Early detection of kidney disease is key to preventing its progression to advanced stages, where treatment options may be limited. Regular screening for kidney function, including blood tests to measure serum creatinine and urine tests to assess proteinuria, can help identify individuals at risk for kidney disease.
In addition to early detection, lifestyle modifications play a vital role in maintaining kidney health and preventing kidney disease. Adopting a healthy lifestyle, including eating a personalized integrative diet, staying physically active, maintaining a healthy weight, avoiding smoking, and limiting alcohol consumption, can help reduce the risk of developing kidney disease and its complications.
The bottom line on the World Kidney Day 2024
As we observe World Kidney Day 2024, let us reaffirm our commitment to advancing kidney health for all. By promoting equitable access to care, optimizing medication practices, and emphasizing early detection and prevention, we can work towards a future where everyone has the opportunity to enjoy optimal kidney health. Together, let us strive to create a world where kidney health is a priority for all, regardless of background or circumstance.
Early Detection of Chronic Kidney Disease: A Key to Preserving Health and Preventing Progression
Chronic Kidney Disease (CKD) is a progressive condition where the kidneys lose their ability to filter blood effectively over time. This can lead to a buildup of waste products in the body, causing various health issues. Early detection of CKD is crucial for several reasons, including slowing disease progression, improving quality of life, and reducing the risk of complications. This blog post will delve into the importance of early detection of chronic kidney disease, how it can be achieved, and the potential outcomes of timely intervention.
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding chronic kidney disease
Chronic Kidney Disease is often silent in its early stages, making it particularly dangerous. Many individuals do not experience symptoms until their kidney function has significantly declined. CKD is commonly caused by diabetes and high blood pressure, which can damage the kidneys' filtering units, leading to reduced kidney function. This is followed by autoimmune kidney diseases (glomerulonephritis).
Understanding the Stages of Chronic Kidney Disease
Chronic Kidney Disease is categorized into five stages based on the degree of kidney damage and function. These stages are determined by measuring the glomerular filtration rate (GFR), which assesses how well the kidneys are cleaning the blood.
- Stage 1: Kidney damage with normal or high GFR (≥90 mL/min/1.73 m²). At this stage, the kidneys are still functioning well, but evidence of kidney disease is detected through other means, such as protein in the urine or physical damage to the kidneys.
- Stage 2: Mild loss of kidney function (GFR of 60-89 mL/min/1.73 m²). Kidney damage is more apparent, but the kidneys still function reasonably well.
- Stage 3: Moderate loss of kidney function, subdivided into stages 3A (GFR of 45-59 mL/min/1.73 m²) and 3B (GFR of 30-44 mL/min/1.73 m²). Symptoms may become more noticeable, including fatigue, fluid retention, and changes in urination patterns.
- Stage 4: Severe loss of kidney function (GFR of 15-29 mL/min/1.73 m²). At this stage, the damage is more severe, and the risk of kidney failure increases. Symptoms can include more significant fluid retention, urinary changes, and other complications affecting overall health.
- Stage 5: Kidney failure (GFR <15 mL/min/1.73 m²) or end-stage renal disease (ESRD). At this final stage, the kidneys have lost nearly all their ability to function effectively, requiring dialysis or kidney transplantation for survival.
Early detection of CKD, ideally in stages 1 or 2, allows for interventions that can slow the progression of the disease and significantly reduce the risk of advancing to end-stage renal disease. Understanding these stages emphasizes the importance of regular screening and monitoring for those at risk of CKD.
Join us to end the kidney disease epidemic
The Importance of early detection
Slowing disease progression
Early detection of CKD allows for interventions that can slow the progression of kidney damage. By managing underlying causes, such as diabetes and hypertension, individuals can significantly reduce the rate at which kidney function declines. In fact, I truly believe that early intervention with lifestyle modifications and an integrative approach can halt or even reverse CKD.
Reducing complications
CKD can lead to complications, including cardiovascular disease, anemia, bone disease, and electrolyte imbalances. Early detection and management of CKD can help prevent or mitigate these complications, improving overall health outcomes and quality of life.
Enhancing quality of life
Early intervention in CKD can significantly enhance the quality of life for individuals with the disease. By managing symptoms and complications early, individuals can improve their lifestyle to maintain a more active and fulfilling life despite their condition.
Economic benefits
The early detection of CKD can also have significant economic benefits. By reducing the need for more intensive treatments and managing complications early, healthcare costs associated with advanced CKD and its treatments can be significantly reduced.
Indeed, current models for early detection of CKD suggest that screening individuals at high risk, such as those with hypertension or diabetes, is economically sound. This is based on cost-effectiveness thresholds ranging between $50,000 and $150,000 USD per quality-adjusted life year gained.
How to achieve early detection
Early detection of CKD involves regular screening for individuals at risk, including those with diabetes, hypertension, a family history of kidney disease, or other risk factors. Screening tests typically include blood tests for serum creatinine (used to estimate glomerular filtration rate (GFR)), and urine tests for albumin (a protein that can indicate kidney damage).
The Kidney Disease: Improving Global Outcome (KDIGO) recommends screening for early detection of CKD in these individuals:
- Persons with hypertension, diabetes, or cardiovascular disease.
- Other high-risk individuals or populations based on comorbidities, environmental exposures, or genetic factors.
KDIGO also recommends utilizing the combination of measurements of serum creatinine, serum cystatin C, and urine albumin as the standards for early detection of CKD.
Encouraging at-risk populations to get screened
Awareness campaigns and healthcare policies should focus on encouraging at-risk populations to undergo regular screening for CKD. This can involve educational efforts to inform the public about the risks of CKD and the benefits of early detection.
Integrating screening into primary care
Integrating CKD screening into routine primary care visits can help ensure that individuals at risk are identified early. Primary care providers play a crucial role in identifying at-risk individuals and initiating screening and follow-up care.
At-home testing for early detection of CKD
At-home testing for the early detection of CKD offers a convenient and accessible way for individuals to monitor their kidney health, especially those at high risk due to conditions like diabetes or hypertension. These tests typically involve the analysis of a urine sample for markers such as protein or albumin, which can indicate kidney damage.
Quality at-home measurements of BUN and creatinine have recently become commercially available and affordable. This testing allows for the estimation of GFR and early detection of the decrease in kidney function.
By facilitating early detection, at-home tests can play a crucial role in managing and slowing the progression of CKD, allowing for timely medical intervention and lifestyle adjustments.
Get a 25% discount on Kidney testing with promotional code IKI25.
The bottom line on early detection of chronic kidney disease
Early detection of chronic kidney disease is critical to managing the disease and improving outcomes for individuals at risk. Through regular screening, management of underlying conditions, and early intervention, the progression of CKD can be slowed (or even halted), complications can be reduced, and the quality of life for those with the disease can be significantly improved. Healthcare providers, policymakers, and individuals must work together to prioritize early detection and management of CKD to achieve these goals.
February Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the February Research and News.
Self-Reported Frequency of Adding Salt to Food and Risk of Incident Chronic Kidney Disease
In a comprehensive cohort study leveraging UK Biobank data, researchers investigated the potential link between the self-reported frequency of adding salt to foods and the risk of developing chronic kidney disease (CKD) among adults.
This population-based research tracked participants aged 37 to 73 years, free from CKD at the start, from 2006 to 2010, with follow-up for disease diagnosis extending into April 2023.
Participants' salt-adding habits were categorized into four groups: never or rarely, sometimes, usually, and always. The study aimed to discern if these self-reported habits correlated with an increased incidence of CKD, adjusting for a wide range of potential confounders like demographic factors, health status, and lifestyle choices.
Over the median follow-up of 11.8 years among 465,288 individuals, those with a higher frequency of adding salt reported at baseline showed a statistically significant increase in CKD risk, adjusted for various health and lifestyle factors.
Notably, the risk of CKD incrementally rose with the frequency of adding salt to food, with those always adding salt at the table showing the highest risk. Additionally, the associations were more pronounced among participants with a higher estimated glomerular filtration rate (eGFR), lower body mass index (BMI), or lower physical activity levels.
Why this is important
This study underscores the potential public health impact of seemingly benign dietary habits, such as adding salt to foods, on the risk of developing chronic kidney disease. By establishing a link between higher self-reported salt addition and increased CKD risk, the findings advocate for dietary modifications as a preventive measure against CKD in the general population.
Reducing the frequency of adding salt at the table could serve as a simple yet effective strategy to mitigate CKD risk, particularly highlighting the importance of dietary interventions alongside other lifestyle modifications to preserve kidney health.
But the risk probably extends beyond added salt to ultra-processed foods that often contain high amounts of salt.
Join us to end the kidney disease epidemic
Social Isolation and Loneliness Increase the Risk of Acute Kidney Injury in Adults
In a prospective cohort study utilizing the UK Biobank data, investigators explored the impact of social isolation and loneliness on the risk of developing acute kidney injury (AKI) among middle-aged and older adults.
Including 450,868 participants without prior AKI, the study constructed a social isolation index based on living conditions, frequency of social contacts, and participation in social activities. Loneliness was evaluated through self-reports.
Over a median follow-up of 12 years, 18,679 participants (4.1%) developed AKI, with the majority of cases identified through hospital admission records. The study found that social isolation significantly increased the risk of AKI, with a hazard ratio of 1.50 in minimally adjusted models and 1.10 in fully adjusted models that accounted for a comprehensive range of factors, including socioeconomic status, health behaviors, and psychological factors.
Similarly, loneliness was associated with a higher risk of AKI, with hazard ratios of 1.57 in minimally adjusted models and 1.10 in fully adjusted models. Notably, the highest risk of AKI was observed in individuals experiencing both social isolation and loneliness, with specific factors such as living alone and limited social contact being significant predictors of AKI risk.
Why this is important
This study highlights the significant and independent associations between social isolation, loneliness, and the risk of AKI, underscoring the broader health implications of social determinants.
Understanding that social isolation and loneliness can contribute to the risk of serious health conditions like AKI emphasizes the need for healthcare providers and policymakers to consider social factors in preventative health strategies.
These findings advocate for interventions aimed at enhancing social connections and addressing loneliness as potential measures to reduce AKI risk, illustrating the interconnectedness of social well-being and physical health.
Impact of Potassium Citrate Treatment Differs Depending on Type of Calcium Stone
In a detailed analysis, researchers investigated how common treatments for calcium stones—specifically thiazide diuretics and potassium citrate (K-Cit)—affect patients with calcium oxalate (CaOx) and calcium phosphate (CaP) stones.
The study assessed the impact of these treatments by examining changes in 24-hour urine compositions across four treatment groups: Lifestyle changes, K-Cit, Thiazide, or a combination of Both medications.
The findings revealed that thiazide treatment effectively reduced urine calcium levels in both CaOx and CaP stone formers, while K-Cit did not significantly alter urine calcium.
In CaOx stone formers, urine citrate levels increased with K-Cit treatment, a response not mirrored in CaP stone formers. Interestingly, urine pH increased in all CaOx stone formers but only in CaP stone formers treated with K-Cit.
Supersaturation (SS) levels for CaOx decreased in patients treated with either Thiazide or K-Cit, while CaP SS decreased in both stone groups treated with Thiazide but not in CaOx stone formers treated with both medications. K-Cit treatment alone led to an increase in CaP SS in CaOx stone formers.
Why this is important
This study highlights the differential impacts of thiazide diuretics and potassium citrate on urine stone risk factors in calcium oxalate versus calcium phosphate stone formers. While Thiazide universally lowers urine calcium and stone risk for both types of stone formers, potassium citrate benefits appear more limited to CaOX stone formers.
These findings underscore the need for tailored medical approaches in managing calcium stone diseases, suggesting that the effectiveness of K-Cit in preventing stone recurrence may vary significantly between CaOx and CaP stone formers. This insight is crucial for clinicians in optimizing treatment strategies and improving outcomes for patients with these common types of kidney stones.
The Type of Plant-Based Diets Matters When it Comes to Mortality and CKD Progression Risk
In a prospective analysis within the Chronic Renal Insufficiency Cohort (CRIC) Study, investigators explored how adherence to plant-based diets impacts the progression of chronic kidney disease (CKD) and all-cause mortality among CKD patients.
The study, involving 2,539 CKD participants enrolled from 2003 to 2008, utilized dietary responses to calculate adherence scores to "overall," "healthy," and "unhealthy" plant-based diet indices.
What defined a healthy and unhealthy plant-based diet in the study?
Their scoring index defined a healthy plant-based diet as a diet containing whole grains, fruits, veggies, nuts, legumes, tea, and coffee. An unhealthy plant-based diet contains refined grains, potatoes, fruit juices, sweetened beverages, sweets, and desserts. You can check it out here.
Results over median follow-up periods of 7 and 12 years for CKD progression and mortality, respectively, revealed that higher adherence to overall and healthy plant-based diets significantly reduced all-cause mortality risk by 26% and 21%.
Conversely, a 10-point increase in the score for unhealthy plant-based diets modestly heightened the risk for both CKD progression and all-cause mortality.
Why this is important
These findings underscore the potential benefits of plant-based diets in managing the progression of CKD and reducing mortality risk. Highlighting the distinction between healthy and unhealthy plant-based diets, this study advocates for dietary guidance focused on nutritious plant foods versus processed plant foods for CKD patients.
By demonstrating a clear link between diet quality and health outcomes in CKD, this research provides a strong foundation for dietary recommendations to improve long-term health and survival in this population.
Review article of the month
The Role of Inflammation in Salt-Sensitive Hypertension
Salt sensitivity of blood pressure (SSBP) stands as an independent risk factor for cardiovascular diseases. The epithelial sodium channel (ENaC) is pivotal in regulating renal electrolytes and fluid balance and is involved in developing SSBP. This review highlights the latest findings on how ENaC-related inflammation contributes to the onset of SSBP.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Five Benefits of Cold Showers for Kidney Health
Cold showers have gained popularity for their invigorating effects on health and well-being. Incorporating cold showers into your daily routine can offer several specific health benefits, particularly for individuals with kidney issues. This blog will discuss the five benefits of cold showers for kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
How to take cold showers for kidney health
The benefits of a cold shower begin when the water temperature dips to 60 degrees Fahrenheit. This is 40 degrees lower than the typical steamy shower.
Now, I know it will be hard to turn that shower knob from hot to cold in the morning. The way to do it is to start with your hot shower. Then gradually lower the temperature at the end of the shower every day by turning the knob to cold.
Give your body time to adjust. You can start with 30 seconds under the cold and gradually increase it to a maximum of three minutes. A quick shower for kidney health takes 10 minutes.
Join us to end the kidney disease epidemic
The benefits of cold showers for kidney health
Enhanced Circulation
Boosting Blood Flow: Cold water exposure can stimulate blood circulation. The cold temperature causes blood vessels to constrict and then dilate, which can help improve overall blood flow. Enhanced circulation is beneficial for kidney health as it ensures efficient delivery of nutrients and removal of wastes from the kidneys.
Reduced Inflammation
Natural Anti-Inflammatory: Cold showers can act as a natural anti-inflammatory agent. The cold temperature helps in reducing swelling and edema, which is particularly useful in managing conditions like kidney disease, where inflammation is common.
Stress Reduction
Lowering Stress Hormones: Cold showers can trigger a decrease in stress hormones, like cortisol. This reduction in stress is beneficial for overall health and can indirectly support kidney function by maintaining a balanced hormonal environment in the body.
Immune System Boost
Strengthening Immunity: Regular cold showers can enhance the immune system's efficiency. A robust immune system is vital for kidney patients, as it helps in fighting infections and reduces the risk of complications related to kidney disease.
Improved Sleep Quality
Enhancing Sleep Patterns: Cold showers can improve sleep quality by normalizing the body's temperature regulation system. Good sleep is crucial for kidney health, as it allows the body to repair and regenerate, providing the kidneys the rest they need to function optimally.
More benefits of cold showers for kidney health
Cold exposure, such as cold showers, has many other benefits for kidney patients. It increases endorphins and fights off depression. It improves metabolism and circulation. Most importantly, it stimulates the vagus nerve and improves renal circulation. It may also soothe itchy skin which is common in CKD patients. In addition, cold showers help with post-workout muscle soreness.
The bottom line
Incorporating cold showers into your daily routine can offer multiple health benefits, particularly for those with kidney issues. However, it’s essential to approach this practice with caution and always consult with your healthcare provider before making significant changes to your routine, especially if you have underlying health conditions. With the right guidance, cold showers can be a refreshing and health-boosting addition to your kidney care regimen.
The Increasing Concern of Microplastics on Kidney Health: A Closer Look
In recent years, the ubiquity of microplastics in our environment has become a growing concern, particularly regarding their potential impact on human health. Microplastics, tiny plastic particles less than 5mm in size, have been found in various everyday items like water bottles, food packaging, and even in the air we breathe. In this blog, we will discuss the effects of microplastics on kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Understanding microplastics: A deep dive
Microplastics are defined as small plastic pieces less than five millimeters long, which can be harmful to our ocean and aquatic life. These tiny pollutants originate from a variety of sources, including larger plastic debris that degrades into smaller and smaller pieces.
Additionally, microplastics are directly released into the environment in the form of microbeads from beauty products and microfibers from synthetic clothing. Due to their small size, microplastics can easily bypass water treatment plants, leading to widespread pollution in water bodies, including oceans, rivers, and lakes.
The pervasiveness of microplastics in our environment raises significant concerns due to their potential to enter the food chain. Marine animals often ingest these particles, and through bioaccumulation, microplastics can move up the food chain to humans.
Studies have shown that microplastics are found in common foods and beverages, such as seafood, salt, and bottled water, posing a risk to human health. Their presence in the human body has been linked to various health issues, including inflammation and potential cell toxicity, highlighting the urgent need for strategies to reduce microplastic pollution and exposure.
Join us to end the kidney disease epidemic
Hidden sources of microplastics
Hidden sources of exposure to microplastics include tea bags, bottled water, cosmetics with microbeads, synthetic clothing fibers released during washing, seafood contaminated with microplastics, and dust that settles on food and surfaces. These sources contribute to the inadvertent ingestion and inhalation of microplastics by humans, posing potential health risks.
This study, for example, highlights that plastic chopping boards, particularly those made from polypropylene and polyethylene, are significant sources of microplastic contamination in food. The release of microplastics varies with the material of the chopping board and the chopping style, with polypropylene boards releasing more microplastics than polyethylene.
Additionally, the presence of vegetables like carrots during chopping can increase microplastic release from polyethylene boards. Annual human exposure to microplastics from these sources can be substantial, though preliminary toxicity tests on mouse cells did not show immediate adverse effects.
Effects of microplastics on health
Microplastics pose a multifaceted threat to human health, impacting various bodily systems, including the digestive, respiratory, endocrine, reproductive, and immune systems, through ingestion and inhalation.
They can cause inflammation, oxidative stress, endocrine disruption, and neurotoxicity, affecting the body's metabolic processes and leading to various diseases.
Despite the clear indications of harm in cellular and animal studies, comprehensive epidemiological research is needed to fully understand the implications of microplastics on human health and establish safe exposure levels.
Effects of microplastics on kidney health
Studies on the effects of microplastics on kidney health come from laboratory models. For example, this study showed that exposure to microplastics caused mitochondrial dysfunction, inflammation and cell death in human and mice tubular cells.
Most of the research on the impact of microplastics on kidney tissues was published in the last three years. Studies highlight their potential nephrotoxicity through inflammation, oxidative stress, and lipid metabolism disturbance.
Studies show microplastics can cause cellular and tissue damage, including mitochondrial stress and autophagy in kidney cells. Chronic exposure to microplastics, often combined with other pollutants, exacerbates kidney damage through oxidative stress, apoptosis, and fibrosis.
Practical steps to minimize the exposure to microplastics
To minimize exposure to microplastics, consider the following steps:
- Opt for natural fiber clothing and textiles.
- Use glass or stainless steel containers instead of plastic.
- Avoid single-use plastics.
- Choose cosmetics and personal care products without microbeads.
- Prefer glass or stainless steel bottles for drinking.
- Prepare meals with fresh ingredients to avoid packaged foods.
- Avoid plastic cutting boards.
- Use a high-quality water filtration system that is tested to remove microplastics.
The bottom line on the effects of microplastic on kidney health
The presence of microplastics in our environment and their potential to harm kidney health cannot be overlooked. Chronic exposure to microplastic has been found to damage kidney cells. It's essential to continue research in this area and to consider ways to reduce our exposure to microplastics. By making informed choices about our products and advocating for policies limiting plastic pollution, we can help protect our kidneys and overall health from the unseen dangers of microplastics.
Living Well with Kidney Disease: Kidney Disease Lifestyle Management
Living with kidney disease often means adapting to a new lifestyle that supports kidney health and overall well-being. Think of it as “kidney disease lifestyle management.” By incorporating a few key habits into your daily routine, you can significantly improve your quality of life. Here’s a guide to a day in the life of someone managing kidney disease, focusing on morning rituals, stress reduction techniques, evening routines, and more.
By Majd Isreb, MD, FACP, FASN, IFMCP
Kidney Disease Lifestyle Management - Living Well with Kidney Disease
Morning routine for kidney health
- Hydration (With a Caveat): Starting your day with a glass of water is generally a healthy practice. However, if you have kidney disease, it's crucial to follow your doctor's advice regarding fluid intake. This advice varies depending on the stage of your kidney disease and any fluid restrictions you may have.
- Gentle Exercise: Morning is a great time for light exercise. This could be a short walk, yoga, or stretching. Exercise helps in managing blood pressure and weight, both crucial for kidney health.
- Journaling: Keeping a journal can be therapeutic. It helps in managing stress and anxiety, which is especially beneficial for those with chronic health conditions.
- Meditation and Mindfulness: Starting your day with meditation can help center your thoughts and reduce stress, improving overall mental health.
- Cold Showers: Although it might seem daunting, a cold shower can invigorate your system and boost circulation. However, it’s important to consult with your healthcare provider before making this a part of your routine.
Join us to end the kidney disease epidemic
Light therapy and its benefits
Light therapy typically involves a light box emitting bright light that mimics natural outdoor light. It's a recognized treatment for various conditions, including sleep disorders, depression, and certain circadian rhythm disruptions.
Benefits for Kidney Patients
- Regulating the Sleep-Wake Cycle: For kidney patients, maintaining a regular sleep-wake cycle is crucial. Light therapy in the morning can help synchronize the body's internal clock, leading to better sleep quality. This is especially important since kidney disease can often disrupt normal sleep patterns.
- Improving Sleep Quality: Adequate exposure to light during the day, particularly in the morning, can enhance the production of melatonin in the evening. Melatonin is a hormone that plays a key role in sleep. By increasing melatonin production, light therapy can improve the onset and quality of sleep.
- Boosting Mood and Cognitive Function: Patients with kidney disease often experience mood fluctuations and cognitive changes. Light therapy has been shown to alleviate symptoms of depression and improve cognitive function, which can be beneficial for overall mental health.
- Managing Fatigue: Fatigue is a common symptom in those with kidney disease. Regular light therapy can help reduce feelings of tiredness and increase energy levels, contributing to a more active and engaged lifestyle.
Implementing Light Therapy in Daily Routine
- Timing: The most effective time for light therapy is usually in the morning. About 20-30 minutes of exposure to a 10,000-lux light box is often recommended.
- Consistency: Daily use, especially during the winter months or in less sunny climates, can provide the most benefits.
- Consultation with Healthcare Providers: It’s important for kidney patients to discuss light therapy with their healthcare team, especially if they have other conditions like eye diseases or take medications that increase light sensitivity.
Stress reduction techniques
Chronic kidney disease can be stressful. Techniques like deep breathing exercises, progressive muscle relaxation, or engaging in a hobby can help in managing stress levels.
Deep Breathing and Progressive Muscle Relaxation:
- Deep Breathing: Simple deep breathing exercises can quickly induce a state of calm, lowering heart rate and blood pressure, crucial for stress management.
- Progressive Muscle Relaxation (PMR): This technique, involving tensing and relaxing different muscle groups, helps identify and release physical tension, leading to reduced stress levels.
Engaging in Hobbies and Mindfulness:
- Hobbies: Participating in activities that bring joy, such as crafting, music, or gardening, can offer therapeutic benefits and a welcome distraction from daily stressors.
- Mindfulness and Meditation: Regular mindfulness practices, including meditation, enhance emotional regulation and help in staying grounded and calm.
Evening rituals to improve sleep
A calming evening routine is crucial for good sleep hygiene. This can include:
- Screen-Free Time: Reducing exposure to screens an hour before bed helps in winding down.
- Relaxing Activities: Consider reading, listening to soft music, doing some gentle stretching, or taking a warm bath.
- Creating a Restful Environment: Ensure your bedroom is comfortable, cool, quiet, and dark.
Enhance your sleep quality with the 3-2-1 approach, a simple yet effective routine to prepare your body and mind for restful slumber.
This method advocates for a gradual wind-down by setting specific timeframes: cease consuming any food or alcohol 3 hours before bedtime to aid digestion and avoid disruptions in sleep patterns; halt all work-related tasks 2 hours before sleep to allow your mind to relax and transition away from the stresses of the day; and finally, eliminate screen time at least 1 hour before bed, reducing exposure to stimulating blue light that can interfere with the natural sleep-wake cycle.
Adopting this structured approach can significantly improve your overall sleep quality, leading to a more refreshed and energized you.
Nutrition: A brief note
While nutrition is a complex topic that deserves its own discussion, remember that a balanced diet plays a vital role in managing kidney disease. Opt for kidney-friendly foods and consult with a dietitian for personalized advice.
Avoiding toxins
It’s crucial to avoid toxins that can further harm the kidneys. This includes environmental toxins, tobacco, and alcohol. Maintaining a toxin-free lifestyle supports kidney function and overall health. We provided links to other blogs that discussed these in detail.
Regular check-ups and monitoring
Regular visits to your healthcare provider to monitor kidney function and overall health are essential. This helps in making timely adjustments to your treatment plan.
The bottom line
Living with kidney disease requires a holistic approach to health. By incorporating these lifestyle changes, you can improve your kidney health and overall well-being. Remember, each person's journey with kidney disease is unique, so it's important to tailor these suggestions to your specific needs and always consult with your healthcare provider before making any significant changes to your routine.
Understanding the Gut-Kidney Axis in IgA Nephropathy: A New Perspective
IgA Nephropathy (IgAN), a common form of kidney disease, has been a focus of recent medical inquiries. Recent studies, however, have illuminated a critical aspect: the important role of the gut-kidney axis in IgA nephropathy. This discovery not only deepens our understanding of IgAN but also paves the way for innovative treatment approaches.
By Majd Isreb, MD, FACP, FASN, IFMCP
A primer on the nephron
The nephron, the kidney's fundamental filtering unit, comprises two main parts: the glomeruli and tubules. The glomerulus (pleural glomeruli), first, is a compact yet intricate structure formed by a network of small blood vessels. These vessels are lined with "endothelial cells," which, along with cells called podocytes, create a selective barrier. This barrier filters blood, retaining larger molecules and cells. Mesangial cells provide structural support and regulate blood flow within the glomerulus.
Join us to end the kidney disease epidemic
Understanding IgA nephropathy
IgA nephropathy is an autoimmune disorder predominantly affecting mesangial cells. The disease process begins when the immune system erroneously produces abnormal IgA proteins. These proteins, perceived as foreign, instigate an immune response. Immune complexes formed in this process accumulate in the kidneys, attaching to mesangial cells, leading to inflammation and kidney tissue damage.
The 'four-hit' hypothesis of IgAN
The pathogenesis of IgAN is encapsulated in the "four-hit hypothesis," which posits:
- Elevated levels of abnormal IgA1 (galactose-deficient IgA1 or gd-IgA1)
- Autoantibody production against this abnormal IgA1
- Formation of immune complexes involving these autoantibodies and CD89
- Deposition of these complexes in the glomerular mesangium, culminating in kidney damage
Significant to IgAN is dimeric IgA1, predominantly originating from the gut's mucosa-associated lymphoid tissue (MALT). In fact, the site of gd-IgA1 production is presumed to be the Peyer’s patches of the gut and mesenteric lymph nodes.
The exact reason for abnormal IgA1 production remains unknown, but genetic and environmental factors likely contribute. This review article offers detailed insights into IgAN's innate and adaptive immune mechanisms. It is freely accessible.
Genetic predisposition in IgA nephropathy
Genome-wide association studies (GWAS) involving over 20,000 patients have identified specific genetic variants linked to IgAN. These variants relate to intestinal mucosal integrity (think leaky gut), inflammatory bowel disease and immune responses to intestinal pathogens.
Epidemiological studies have further underscored the connection between gut disorders and IgAN, suggesting a higher incidence of inflammatory bowel diseases in IgAN patients. Another study found that patients with celiac disease are at more risk for IgAN.
The gut-kidney axis in IgA nephropathy
Emerging research underscores the significant impact of gut health on the kidneys, particularly in the context of IgA Nephropathy. Gut microbiota and their derivatives are crucial in this regard. Gut dysbiosis in predisposed individuals may trigger IgAN.
Studies have identified specific microbiota imbalances in IgAN patients, including Escherichia-Shigella dysbiosis. Another study showed that gut of patients with IgAN have higher levels of Streptococcus and Enterococcus and lower levels of Bacteroidetes and Bacteroides. The study also showed multiple metabolites that are generated from the gut microbiota and are associated with IgAN.
The link between gut microbiota, dysbiosis, and IgAN is further supported by the remission of IgAN after fecal transplant and the improvement of IgAN in mice treated with broad-spectrum antibiotics.
However, the association between increased intestinal permeability and IgAN remains inconclusive. For example, this study showed that increased intestinal permeability is common in patients with various glomerular diseases and not unique to IgAN. While other studies found a significant link between increased IgAN and increased gut permeability.
Finally, healthy gut microbiota produces short-chain fatty acids (SCFAs) that are crucial for the health and integrity of the gut. A recent study looked at the changes in SCFAs in patients with IgAN and found that their levels are much lower in these patients. These levels were associated with clinical activity and gut microbiota.
Dietary triggers in IgAN
Dietary sensitivities are prevalent in IgAN patients, with gluten being a notable trigger due to its link with celiac disease. However, many other food triggers were associated with IgAN. Increased intestinal permeability was documented to precede the IgA immune response to food triggers.
The following foods have been found in different studies to be a possible trigger for IgAN:
- Soy.
- Egg whites.
- Milk protein.
- Gliadin/gluten.
- Oat.
Therefore, testing IgAN patients for celiac disease by testing for tissue transglutaminase-IgA antibodies and total IgA levels or even HLA typing (HLA-DQ2 and DQ8) may be indicated.
Unfortunately, common food sensitivity testsdo not test for IgA antibodies. Therefore, food sensitivity testing using IgE and IgG antibodies may not be sufficient to identify food triggers for a specific patient. Therefore, an elimination diet remains the gold standard for that purpose.
Mesangial cells: Intersection of the gut- kidney axis in IgA nephropathy
Mesangial cells, the primary site for IgAN in the kidneys, have receptors for IgA1 antibodies. Interestingly, the mere presence of abnormal Gd-IgA1 is not sufficient to cause IgAN. Mesangial cells in IgAN patients exhibit increased interleukin-6 production and higher matrix gene expression, indicating a specific predisposition.
Substances from the gut, like toll-like receptor 4 (TLR-4), which rises with gram-negative bacteria dysbiosis, are also found in mesangial cells of IgAN patients. Blocking TLR-mediated signaling could be a promising approach for reversing IgAN. This can be accomplished by berberine, N-acetyl-L-Cysteine, and resveratrol.
Recommended Testing for IgAN
In addition to conventional urine and blood tests, we recommend having the patient undergo an elimination diet for 3-4 weeks, as mentioned above. The following testing might also be helpful to assess for triggers and mediators:
- Food sensitivity profile for IgA and IgG such as the one offered by Vibrant Wellness.
- Comprehensive stool analysis that includes testing for SCFAs such as the one offered by Doctor’s Data.
- Celiac profile such as the one offered by Genova Diagnostics.
- Testing for mediators such as vitamin D, and zinc levels.
The bottom line
The exploration of the gut-kidney axis in IgAN exemplifies the dynamic nature of medical science. By understanding these complex interconnections, we can better approach diseases like IgAN with more integrated and precise treatments.