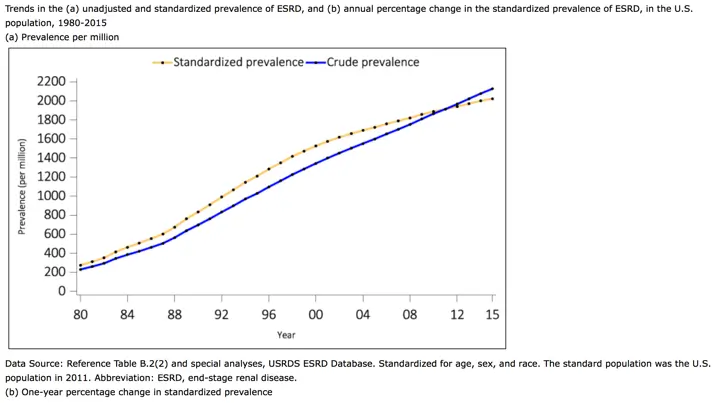
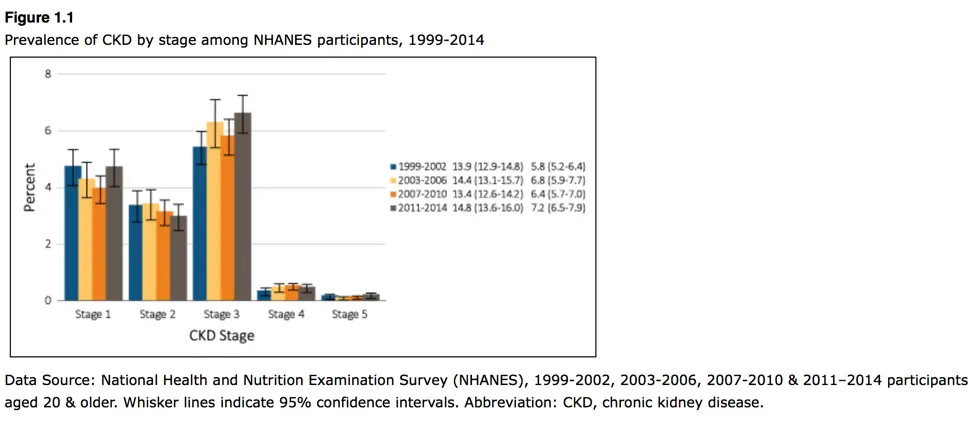
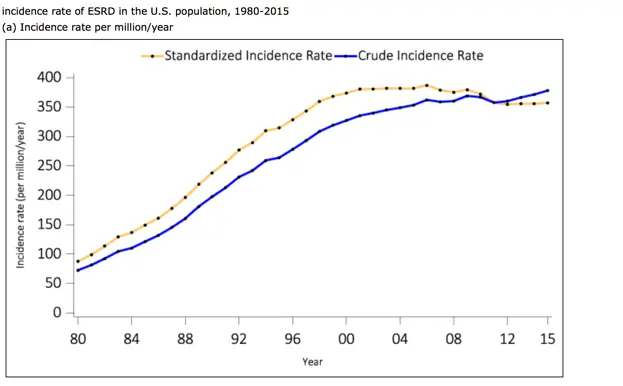
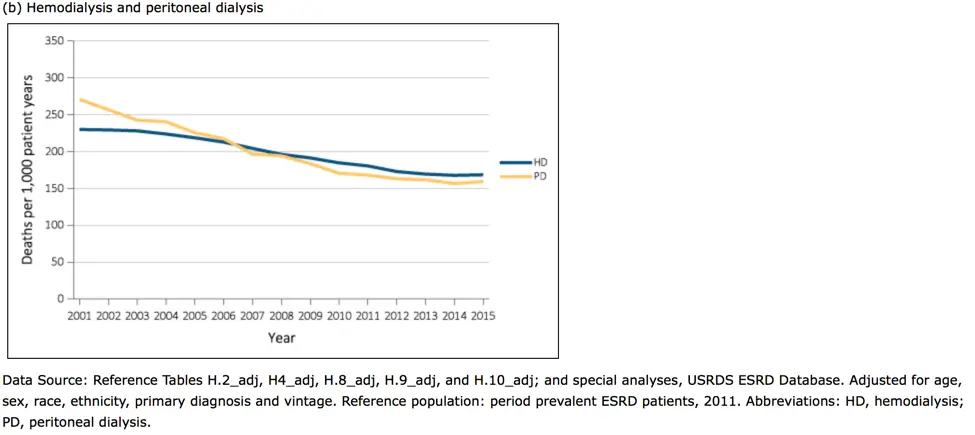
The Gut-Kidney Connection and Autoimmune Kidney Diseases
The gut-kidney axis refers to the relationship between gut integrity and microbiome diversity with kidney disease. The connection between the gut and kidneys is very complex but we can break it down into two major categories: the gut-derived uremic toxins, and the inflammatory immune response that can trigger kidney disease. In this blog, we will focus on the gut-kidney connection as it relates to autoimmune kidney diseases.
The Immune Response: The Firefighters and Sharpshooters
Before we dive into autoimmune kidney diseases, let’s review the basics of the immune system. The immune response can be generally be divided into two arms: innate and adaptive immunity.
Innate Immune response, sometimes referred to as the “primitive” arm, refers to a nonspecific defense mechanism. When a foreign invader, called an antigen, the innate system responds immediately.
The “first responders” of cells that make up the innate system tend to be concentrated around the physical barriers of the body including the skin, gastrointestinal tract, the airways of the lungs. The response is fast and fairly effective, but it’s not very specific so there tends to be some collateral damage. Think of this as the firefighters that work to extinguish a house fire without any regards to the water damage that might impact the house.
Adaptive immune response, on the other hand, is a more targeted immune response. In exchange, this response is more complex than the innate response and takes more time to mount. The antigen’s unique identifying markers is first processed, and the details are committed to memory. When the antigen matches a memory in the database, it’s recognized, and the adaptive immune response triggers a focused immune cells meant to attack and destroy it. This type of adaptive “memory” makes future responses that specific antigen more efficient. You can think of this as a sharpshooter, it takes time, training, and experience, but it’s effective and there’s typically little collateral damage as a result. That said, any problem with the cascade can result in misfiring that turns against the wrong target, as is the case of autoimmune disease.
The GALT
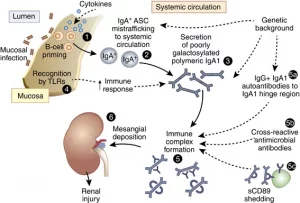
The mucosa of the GI tract has a built-in immune system. In fact, the gut is the largest immune organ in the whole body. This system is called gut-associated lymphoid tissue (GALT) and it is in constant interaction with the intestinal microbiome. The balance of the microbiome is an essential part of managing the GALT, and dysbiosis can trigger a complex immune response involving multiple pathways.
One particular pathway engages what is called the toll-like receptor (TLR) family. These receptors are basically proteins that span the cellular membrane of the many immune cells in the gut and act like scanners. In humans, there are 11 members of this receptor family. Of particular interest, TLR-4 and TLR-2 play important roles and are present on the mucosal epithelial cells of the gut.
These receptors are capable of detecting the unique molecular patterns present on the surface of microbes that are presented when there’s intestinal hyperpermeability (leaky gut). TLR-4, for example, can be activated by lipopolysaccharides (LPS) which are released by certain gram-negative bacteria such as Escherichia Coli (E. Coli). TLR-2 receptors are activated by lipoteichoic acid of gram-positive bacteria and yeast.
So, in essence, the gut has an intricate mechanism to detect pathogens and stimulate the necessary response. At the same time, friendly flora also communicates via the GALT and helps to regulate and improve immune response.
The Inflammatory Response
In the presence of LPS, TLR-4 is activated by binding with their receptors on the surface of the cell (CD14). This triggers a cascade of signals inside the cells (including NF-κB, MAPK, and others). This response leads to the formation of inflammatory molecules broadly called cytokines such as tumor necrosis factor-α (TNF-α) and interleukin among others.
In other words, the domino sequence is triggered when the immune system recognizes a pattern it associates with a pathogen and signals to the immune system to take action. Leaky gut increases the odds of immune response because it increases the exposure of the antigen to the immune receptors. This demonstrates an important concept, the overlap between the innate and adaptive immune system. Hyperpermeability of the gut barrier is often a result of “collateral damage” from non-specific innate response to antigens in the GI (food allergies/sensitivities, toxins, and pathogenic bacteria, yeast, or viruses) which then goes on to train and trigger the more sophisticated adaptive response.
Furthermore, genetics play a role in the likelihood of this complex system over or under performing. Minor genetic code alterations such as single nucleotide polymorphisms (SNPs) in protein presentation of various components (including CD14 and TLR-4) can be associated with an increased risk of immune misfiring and increasing incidence of systemic inflammation, insulin resistance, and autoimmune disorders. Basically, genetics load the gun, the environmental influences pull the trigger.
The Autoimmune Response & the Kidney
Keep in mind, TLR-4 are present not only in the gut but also in the systemic immune system, including the brain and the kidneys. In fact, the signaling pathways between the gut microbiome and the kidneys have been well-documented. The activation of this gut-kidney cascade has been critical in the development of many autoimmune disorders such as antineutrophil cytoplasmic antibody associated vasculitis such as Wegner’s granulomatosis. Furthermore, the activation of toll-like receptors has also been associated in systemic lupus erythematosus (SLE) and IgA nephropathy.
The Bottom Line on the Gut-Kidney Connection and Autoimmune Kidney Disease
The gut houses the GALT, an intricate mechanism necessary to identify and eradicate pathogens. In the presence of pathogenic invaders, an inflammatory response is triggered like a cascade of dominos. When triggered, this response in the presence of certain genetic predisposition can be the perfect storm that leads to various kidney-related immune diseases including vasculitis, lupus, and IgA nephropathy.
This is why when we consider the comprehensive approach to kidney health, it is important to address leaky gut and dysbiosis by employing a comprehensive gut restoration protocol in order to manage the progression of autoimmune kidney disorders and ultimately preserve kidney function.
Impact of Climate Change on Kidney Health
When a large number of young agricultural workers in Central America started developing kidney failure without any known risk factors in the nineties, many kidney organizations around the world started sounding the alarm bells. Scientists have been looking for reasons for this in the past two decades. Initially the connection between climate change on kidney health wasn’t a factor taken into consideration.
It has been suggested that it could be due to agricultural chemicals, heavy metal exposure, silica inhalation, infectious diseases, genetic predisposition. Many of these workers regularly work in hot conditions for long hours. More recently, they identified repeated heat stress as a cause and a risk factor for kidney disease.
There is no doubt that the prevalence of kidney disease is rising in the United States (US) and throughout the world. In fact, one in seven people in the US has kidney disease. It is one of the fastest growing causes of death throughout the globe. An estimated 5–10 million people die annually from kidney disease worldwide. Unfortunately, due to poor data, lack of awareness, early detection and access to care these numbers could underestimate the exact burden of kidney disease in the world.
Chronic Kidney Disease of Unknown Origin (CKDu)
The type of chronic kidney disease that affected the agricultural workers in Central America is now called chronic kidney disease of unknown origin (or CKDu). Since the nineties, CKDu has been identified in studies of similar etiologies in Siri Lanka, India, Africa, South America and the Middle East. The common thread is the hot and humid climate.
CKDu does not follow the conventional risk factors for kidney disease and, therefore, it’s challenging to detect early and prevent. It disproportionately impacts areas with underprivileged communities and poor infrastructure. However, it would be a mistake to assume that this problem is limited to developing countries. Acute kidney injury has been reported in agricultural workers exposed to hot conditions in California and Florida.
Primary Impact: Global Temperature & Kidney Injury
It has been documented that global temperature have increased by about 1 degree centigrade (1.8 degrees Fahrenheit) in the past 50-100 years. Scientists agree that these changes have contributed with record heat waves, melting ice caps and rising sea levels, and extreme weather patterns. This pattern is posing significant health risks, some directly and some indirectly.
According to a United Nations report, climate change is expected to exacerbate health problems that already pose a major burden to vulnerable populations including children and the elderly. Climate change has been associated with a rise in many infectious diseases, especially water-borne illnesses like cholera, typhoid, and dysentery. It is also expected to contribute to the chronic disease burden and bring on new health epidemics. Not surprisingly, CKDu is one of these health issues.
Secondary Impact: Kidney Disease, Pollution, Water & Food Security
So how does rising temperature affect kidney health? The evidence points to heat stress and dehydration can result in chronic kidney disease as playing an important role in the epidemic of CKD worldwide. In fact, the progression of kidney injury has been found to worsen with rising core body temperature.
The mechanism seems to be linked with a decrease in adenosine triphosphate (ATP) levels and reduced mitochondria. These energy powerhouses are particularly abundant in the kidneys, and with reduced ATP and mitochondria, oxidative stress and cellular damage increases. Combine that with a diet with low nutrient-density and inadequate antioxidant content to neutralize oxidative stress, and risk of CKD significantly elevates. In laboratory studies, the supplementation of antioxidants prevented rats who were exposed to heat stress from developing kidney injury.
Furthermore, heat has been associated with increased risk of kidney stones and kidney stones are known risk factors for kidney disease. Since the kidneys are major site for the metabolism and elimination of toxins, exposure to toxins such as glyphosate contributes to kidney injury due to oxidative damage. Glyphosate in particular also impacts dysbiosis and gut health, which may be a confounding factor in the equation when we consider the gut-kidney connection.
As droughts become a more frequent occurrence as a consequence of climate change, dehydration from heat exposure and inadequate water consumption can lead to concentration of these toxins and, therefore, amplification of their negative effects.
Another factor to consider is the increase of pollution like heavy metal, plastics, and chemicals like pesticides and herbicides. Contamination of air and soil with pollutants increases inhalation and ingestion through food, including rise of mercury contamination in fish and arsenic in rice for example. These toxins have been associated with the rise in incidence of KDas well as other chronic diseases like diabetes and hypertension.
Last but not least, as climate change impacts food security and farming practices, access to fresh food and produce might be compromised. This may shift consumption to processed foods with less nutrient value, including less vitamins, minerals, phytonutrients, and antioxidants needed to promote healthy kidneys. Increased consumption of processed foods also leads to reduced fiber consumption, which impacts gut health and the microbiome which might be the most significant factor as we’ve discussed in our blog on the gut-kidney connection.
Other Considerations
It may sound like a cliché, but hydration is key. For those in labor industries or who work in agriculture or at increased risk of extended heat and chemical exposure, extra effort should be made to adequately hydrate. Broader public health measure and policy should be put in place to improve worker safety.
The risk increases for those taking medications that:
· Increases risk for dehydration, including diuretics (furosemide, hydrochlorothiazide, etc) or SGLT-2 inhibitors (canagliflozin, dapagliflozin, etc), or
· Decreases circulation to the kidneys, including ACE inhibitors (lisinopril, captopril, etc), angiotensin receptor antagonists (ARBS like losartan, Olmesartan, etc). However, as we mentioned earlier, heat lead to energy depletion in the kidneys and supplementing with antioxidants may further decrease the risk of kidney injury due to extreme heat.
Bottom Line
Rising global temperatures are posing increasing risk for kidney disease and contributing to a worldwide rise in chronic kidney disease. Extended exposure to heat and dehydration can lead to kidney injury and kidney stones. Improved hydration, improved nutrient-density diet, and use of antioxidants maybe be preventive. Other confounding factors cannot be ignored, including increased environmental pollution, factors that impact on gut health, and medications. Although individuals can take steps to reduce our carbon footprint, but broad public health measures must advocate for policy changes that reduce contributions to climate change and the resulting global health impacts.
The 5R Protocol Part 1: Remove
This is part of a series of blogs discussing an individualized comprehensive gut restoration protocol in chronic kidney disease.
The Gut-Kidney Connection
Researchers have established a relationship between gut integrity and microbiome diversity with various chronic diseases, including kidney disease. Increased intestinal permeability, also known colloquially as “leaky gut” has been shown to be at the root of this connection. This gut-kidney relationship is the result of complex biochemical and immune mechanisms.
Past studies have attempted to explore the impact of dietary changes shifting the gut microbiome to help restore the lining of the gut and reduce the resulting inflammation. However, many of the studies in the literature looking at the use of probiotics to reduce uremic load show some limited benefit in reducing the chronic kidney disease (CKD) burden.
But these studies failed to present a comprehensive approach that reveres damage to the gut while simultaneously inoculating the necessary bacteria. This single dimensional approach does not acknowledge all the different factors involved in the gut-kidney Axis.
To assure that the patient is getting a comprehensive gut restoration protocol, all the mechanisms that underlie probiotic use should be addressed. These include modification of microbiota, competitive adherence to the mucosa and epithelium, strengthening of gut barrier and modulation of the immune system.
The 5R gut restoration program addresses these gaps and help reduce the risk of progression of CKD.
This program is designed to address five areas of GI mucosal integrity repair:
1) Remove potential triggers, including polypharmacy, pathogenic organisms, food intolerances, sensitivities and allergies, or toxic exposure.
2) Replace digestive aid to support improved nutrient absorption and metabolism, including digestive enzymes, or agents that promote improved motility and regular bowel movements.
3) Reinoculate provide an environment where good bacteria can thrive and where bad ones cannot.
4) Repair support of the cellular repair process through the above, as well as by providing specific nutritional support for the regeneration of the GI protective barrier.
5) Rebalance lifestyle factors that influence the gut bacteria such as stress, sleep, exercise and relationships and assure ongoing gut health.
Remove
The first step of this protocol focuses on removing any exposures that may be contributing to increase inflammation in the gut. This includes food exposures, toxins, as well as screening for and treating pathogenic bacteria, fungi, parasites, or viruses that maybe disrupting the normal microbiota balance, which may require antimicrobial treatment to eradicate.
First, food known to cause sensitivities and allergies should be eliminated as part of an elimination diet. There are multiple categories of foods that contribute to inflammatory response that disrupts the lining of the gut. For example, gluten has been found to be associated with the development of leaky gut and IgA nephropathy. At the same time, we should be emphasizing the inclusion of key nutrient-dense foods that help to restore gut integrity and reduce inflammation, including antiinflammatory fats, organic fiber and phytonutrient-rich vegetables and fruit.
Typically, an elimination diet removes common food triggers like gluten, dairy, eggs, and soy. Depending on the root of the food reactivity, sometimes the removal or reduction of grains, legumes, FODMAPS, or night-shade vegetables is also necessary.
A nutritionist or clinician trained in implementing the elimination diet can help guide on which foods to eliminate or reduce. The decision may be done empirically, using symptom monitoring to guide the progress. Alternatively, the program can be personalized by using specialized testing for immune response against food can help guide this process.
Second, exposure to environmentally derived toxic substances should also be minimized, this includes mercury, arsenic as well as pesticides and other environmental pollutants whenever possible. We are exposed to toxic chemicals on a daily basis including pesticides in conventionally-farmed food, non-stick cookware, plastic use, and flame retardants and off-gassing released from furniture.
Addressing this aspect involves focusing on consuming organic produce and animal products and addressing environmental sources of toxicity. This topic is covered in more detail in this blog here.
Lastly, remove also entail identifying potential microbial triggers that might be contributing to inflammatory response. As mentioned above this may be “bad” bacteria, viruses, candida, and/or parasites. The presence of these offenders can be detected through symptoms as well as through advanced stool testing that employs a technique called PCR. Once identified, your practitioner can use antimicrobials to help eradicate the pathogenic organism, using prescriptive antibiotics or even herbal antimicrobials if appropriate.
Where to start?
Inflammatory foods
Some foods can be inflammatory or induce allergic reactivity. Furthermore, they may provide an environment that allows for the growth of pathogenic microbes, including low-fiber diets and high sugar diets. Furthermore, depending on your genetics, some foods can even lead to autoimmune conditions that may affect the kidneys. Eliminating food that is known to cause sensitivities varies dependent on the individual and their genetic predisposition.
An elimination diet can help with identifying what food an individual should avoid. This diet involves the removal of foods commonly associated with food sensitivities or immune reactivity. There are many variations of the elimination diet, and it’s very important to work with a nutritionist who can ensure you’re doing the protocol correctly and not missing any essential nutrients.
After a period of removal lasting at least 4 weeks, and assuming improvement in symptoms that suggests improved gut integrity, your integrative or functional medicine provider will work with you on gradual and careful reintroduction of foods to assess if tolerance has improved.
Decrease/Eliminate Exposure to Toxins
It is very hard to eliminate all sources of toxin exposure, but these steps can help minimize it:
1. Water filtration: we discussed options for water filtration in a previous blog.
2. Cookware: gradually eliminate all toxic non-stick cookware from your kitchen.
3. Avoid plastic containers or utensils: gradually switch from plastic to non-plastic containers and utensils.
4. Eat organic when possible: we know that choosing organic food can be expensive. If you are on a budget, there are certain fruits and vegetables that you should buy organic because of their high toxic burden. Visit the Environmental Working Group website to learn about their “Dirty dozen” T. This list gets updated yearly depending on their tests.
5. Be careful of processed drinks and juices. Not only do these contain excessive amounts of sugar, but they can also be a source of heavy metals and toxins because of poor regulations.
Eliminate Pathogens
As discussed above, laboratory analysis of the patient’s stool and serum can help give insight to the extent of intestinal permeability (leaky gut), as well as the status of microbiota. Establishment of baseline status is essential for measuring outcomes at designated milestones to give quantifiable outcomes. A comprehensive stool analysis can be a good start. In addition, a good dietary intake can help identify issues that could be related to food intake to guide this process.
Depending on the results of the individual’s comprehensive stool analysis, certain bacterial or yeast overgrowth can be detected. Also, parasites can sometimes be identified. Any of these should be treated by various medications, dietary changes, and/or herbal supplements as indicated and advised by an integrative or functional medicine provider.
Remove stress
Stress can have a bad influence on digestion and absorption. People who are stressed tend to eat too fast, make poor choices, and may eat too much at various intervals. This can lead to food choices that can lead to feeding the bad bacteria in the gut and poor digestion causing nutritional deficiencies. Furthermore, stress itself can influence epigenetic changes that impact dysbiosis as well as disease expression in general.
Bottom Line
The first step in an individualized comprehensive gut restoration protocol involves the removal of food sensitivities, environmental toxins, pathogenic organisms, stress. It’s important to work with an integrative or functional medicine provider trained to guide patients with elimination diets can help you navigate this successfully.
Next, we will tackle the second “R” in the gut restoration protocol: Replace.
Kidney Associated Drugs-Nutrient Interactions
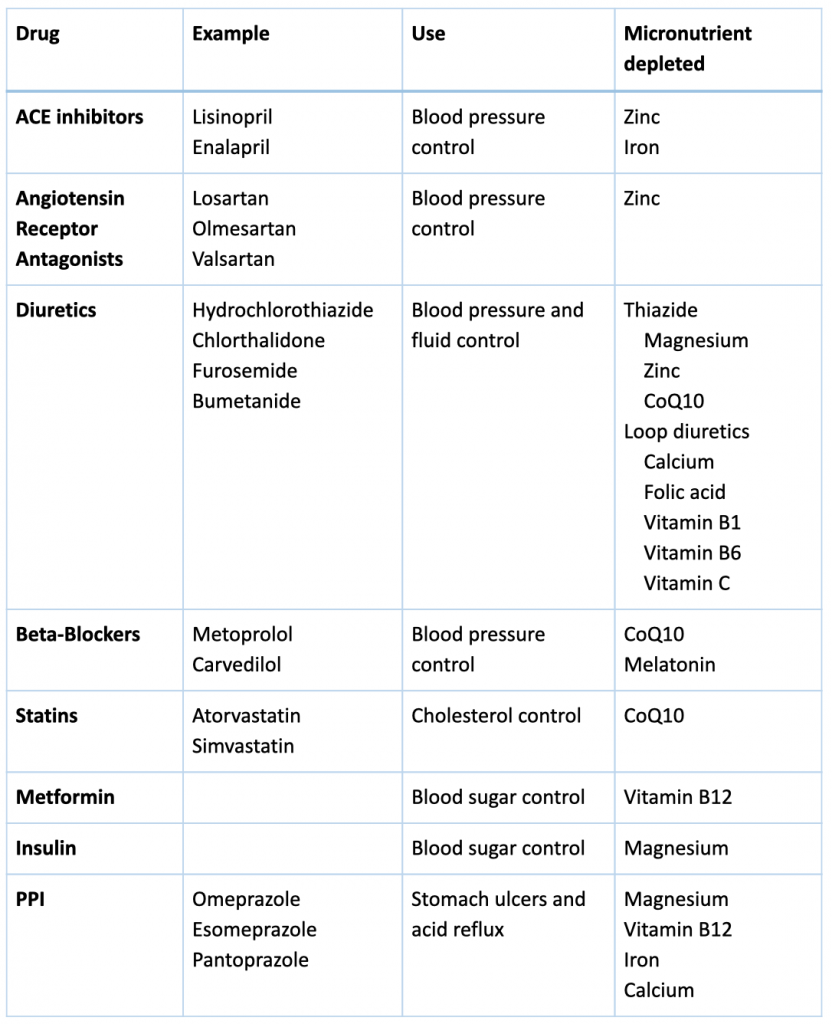
Micronutrients (vitamins and minerals) are essential for every structure and function in the human body, including but not limited to the function of the heart, blood vessels, and immune system. Thousands of enzymes in the body require micronutrients for optimal function. Some medications interact with micronutrients, and micronutrients may interfere with the action of medication. The list of those interactions is long, so we will focus on the effect of those most commonly used in kidney disease.
Angiotensin Converting Enzyme Inhibitors
Angiotensin Converting Enzyme Inhibitors (ACEi) are one of the main classes of blood pressure lowering medications in Kidney disease. ACEi (such as lisinopril) have been found in multiple studies to improve outcomes in diabetic and non-diabetic kidney diseases. However, it’s well documented that ACEi can lead to increased levels of potassium in the blood. This elevation may have many serious effects, especially on the heart and the muscles. Furthermore, supplementing with arginine, a precursor of nitric oxide sometimes used to support blood pressure, while taking an ACEi may further increase the risk of increased blood potassium levels.
The use of ACEi also induces zinc deficiency. By binding zinc, ACEi cause an increase in zinc excretion in the urine. Zinc Deficiency has many negative effects on the body, including cardiovascular and metabolic disease risk.
Angiotensin Receptor Antagonists
Another kidney medications and micronutrients interaction involves angiotensin receptor antagonists (ARBs). Just like ACEi, Angiotensin Receptor Antagonists (such as losartan) are one of the most commonly used blood pressure lowering medications in kidney disease. Like ACEi, ARBs also lead to an increase in potassium so electrolyte balance should be monitored closely. Also similarly to ACEi, evidence suggests that ARBs can also lead to zinc deficiency.
Using the powerful antioxidant alpha-lipoic acid (ALA), which can be used as a supplement, has been found to enhance the effects of ARBs by decreasing inflammation and oxidative stress that leads to cardiovascular risk from atherosclerosis.
Diuretics
Diuretics, also known as water pills, are used in kidney patients for blood pressure and fluid control. They are one of the major causes of kidney medications and micronutrients interactions. There are multiple classes of medications that fall into this broad category, and each affects minerals like potassium, magnesium, calcium and various vitamins differently.
For example, hydrochlorothiazide (HCTZ) and furosemide, can lead to potassium and magnesium deficiency so these nutrients are monitored very closely in patients prescribed these drugs. However, their effect on calcium varies. Thiazide diuretics (such as HCTZ) increase calcium retention while loop diuretics (such as furosemide) induce calcium depletion.
HCTZ can increase zinc loss in the urine leading to zinc deficiency. Furthermore, HCTZ can lead to CoQ10 deficiency (by inhibition of NADH oxidase) an antioxidant compound produced in our cells associated with reduced function of cardiovascular muscle and reduced skeletal muscle strength (more about CoQ10 below under statins).
Loop diuretics, including furosemide, have been found to induce deficiency of folate, vitamins B1, B6, and C . Furthermore, drug-induced calcium loss in the urine associated with this class of diuretics can lead to increased risk of bone loss and increased risk for kidney stones.
Beta-blockers
Beta-blockers (BB), such as metoprolol, are commonly used blood pressure reducing medications because they have been found to improve cardiac outcomes.
However, these medications exert their effect while inhibiting enzymes that are dependent on CoQ10 (including NADH-oxidase and succinoxidase). While these medications do not lead to CoQ10 deficiency per se, the concomitant presence of a CoQ10 deficiency for any other reason can lead to greater inhibition of the heart muscle efficiency as well as leading to worsening heart failure.
In addition, BB (especially metoprolol) have been found to decrease the production of melatonin, a compound naturally produced by the body that acts as an antioxidant and sleep inducer. The production of melatonin in the evening as part of our natural sleep-wake cycle induces sleep, and therefore depletion of melatonin causes sleep disturbances. Poor sleep is a major risk factor for progression of kidney disease, diabetes, and hypertension. In patients taking these medications, melatonin supplementation was found to improve sleep quality.
Statins (HMG-CoA Reductase Inhibitors)
Statins, including atorvastatin and simvastatin among many others, are the most commonly used class of cholesterol lowering medications. One of the major drug-induced nutrient depletions of statins is due to the inhibition of the formation CoQ10. Along with insufficient vitamin D blood levels, depletion of CoQ10 along with certain genetic factors is associated with an increased risk of developing a common side effect referred to rhabdomyolysis (muscle breakdown).
CoQ10 is produced throughout the body in the energy powerhouse of the cells. It is a major part of energy production and metabolic process. ”Co” stands for coenzyme, referencing its action in assisting enzymes in an energy production process called the electron transport chain (ETC). The ETC is an essential part of how energy is derived from carbohydrates and fat. Therefore, depletion of CoQ10 not only increases oxidative stress, but also has a significant effect on the function of every organ in our body, and particularly impact the cardiovascular and renal system. .
In addition,statins have been shown to deplete trace elements, including zinc, copper, and selenium. Furthermore, statins while lowering serum fatty acid concentrations (LDL, TG, etc), they also negatively alter the blood levels of important heart-protective polyunsaturated fatty acids (PUFAs while increasing inflammatory arachidonic acid levels. These drug-nutrient interactions are inconsistent from patient to patient, likely due to genetic variations, agent chosen, and dose. Therefore, monitoring lab and symptoms of deficiency or imbalance is ideal.
Metformin
Metformin is used to improve blood sugar by improving insulin sensitivity. Although some kidney patients might be taking metformin, it is not recommended for use in advanced kidney disease due to the increased risk of lactic acidosis.
Metformin is known to decrease the absorption of vitamin B12 by inhibiting the secretion of intrinsic factor, a compound needed to absorb B12 from food. B12 is a water-soluble vitamin and is essential for many processes, including energy production, production of endogenous proteins used for various cell functions including DNA repair, production of blood cells, myelin (cells that make up the nervous system), and some antioxidants. Therefore, supplementing vitamin B12 in patients taking metformin may prevent anemia, maintain brain health, cell production, and support the detoxification pathways.
Insulin
Insulin which is injected to lower blood sugar in diabetics can lead to significant loss of magnesium in the urine. Low magnesium can lead to significant effect on vascular, bone and heart health. In fact, supplementing magnesium in diabetics can improve insulin response.
Proton Pump Inhibitors (PPIs)
Proton Pump Inhibitors (PPIs) are one of the most commonly used drug classes globally, including in patients with kidney disease. Drugs belonging in this class include omeprazole and pantoprazole, which are commonly used for “acid reflux”, decrease stomach acid production leading to nutrient malabsorption and dysbiosis. They can lead to a decrease in calcium, iron, magnesium and vitamin B12. It’s also well-established that they also directly contribute to kidney injury. Despite that, their use remains common and we should remain vigilant in general.
Bottom Line
Many kidney medications can lead to micronutrient deficiencies with negative effect on outcomes and organ functions. The following table helps summarize these for you with general suggestions for supplementation. We recommend that you work with your integrative medicine provider to address complementary therapy to optimize you treatment and nutritional status.
Comprehensive Gut Restoration and Progression of CKD
This is part one of a series of blogs discussing comprehensive gut restoration protocol and the progression of chronic kidney disease. Gut restoration and progression of CKD.
The Gut-Kidney Connection
Chronic kidney disease affects millions of American and is often associated with comorbid conditions such as cardiovascular disease, diabetes and hypertension. Dysbiosis and leaky gut are implicated in many systemic inflammatory and immune-related factors that lead to chronic disease. Dysbiosis is the term used to define alterations in the gut microbiota that includes overgrowth of bad bacteria, as well as underrepresentation of good bacteria. The normal integrity of the gut can be compromised resulting in systemic complaints, even in the absence of overt gut symptoms.
Gut Restoration and Progression of CKD
Research has established a relationship between gut microbes and different diseases, including kidney disease. This occurs through very complex biochemical and immune mechanisms. The presence of good bacteria has been associated with immune changes that inhibits inflammation. The use of probiotics and prebiotics that produce favorable changes in the microbiome have also been associated with gut changes that reduce advanced glycation end products (AGE), a uremic toxin associated with advancing kidney damage.
So, balancing the gut bacteria help slow kidney disease by directly inhibiting the immune response; and indirectly by reducing toxic burden known to progress CKD.
The Gut as a Potential Source of Inflammation
In the cases of dysbiosis, we see a rise in inflammatory markers that interact with the lining of the gut and result in damage and increased permeability or leaky gut. This causes shifts in the breakdown of nutrients, including amino acids. These shifts increase the levels of circulating substances involved in kidney disease and inflammation such as p-cresol, phenol, and indole. On top of that, these “pro-inflammatory” changes has also been associated with hypertension and increased risk of diabetes and cardiovascular disease; all of which are associated with CKD risk.
Yet, there have been inconsistencies in studies evaluating the benefits of targeting the gut-kidney axis with probiotics and prebiotics. Some suggest this may in part be due to the fact that research in this field is still in its infancy stage and conflicts in research may be due to statistical problems or inadequate interventions. But in an attempt to isolate the intervention, most studies utilized unilateral approach of inoculating the microbiome. In doing so, they usually fail to address other aspects of gut restoration and mucosal repair.
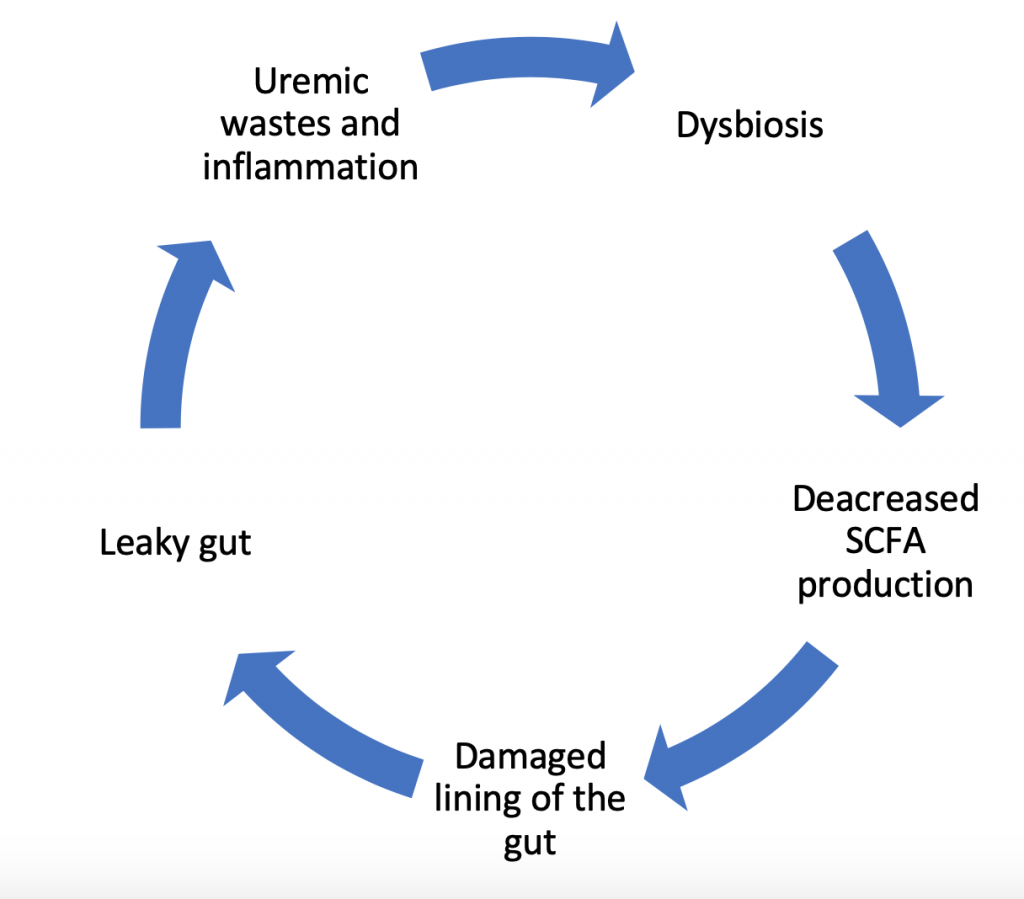
The Dysbiosis Cycle
In fact, changes in the gut bacteria do not operate as a one-way inflammatory force ending in damage to the lining of the gut. Instead it’s a dynamic process with a variety of contributing factors. Research confirms that it’s not only the dysbiosis that causes inflammatory damage, but also damage to the lining of the gut contributes to further dysbiosis and reduced production of short chain fatty acids (which feeds the lining of the gut). This, in-turn, increases the uremic waste products and further perpetuates growth of bad bacteria. This dysbiosis cycle damages the gut cell wall and promotes leakage of parts of the bad bacteria and toxins produced by these bacteria into the bloodstream. This leads to more inflammation, and the cycle repeats.
What is Missing in Current Research?
Individual Factors
As mentioned above, studies focusing on the use of probiotics to improve gut health and reduce inflammation and CKD risk have had mixed results. But these studies looked at a single change to see if it impacts kidney disease when in reality the process is more complex. Considerations for other factors that contribute to inflammation and CKD risk must also be accounted for. This includes environmental exposures, genetic risk factors, metabolic factors and changes in the body fluid volume that contribute to diabetes and hypertension, respectively, contributing to increased CKD risk.
Medications
On top of that, the effect of medication and polypharmacy on the gut and nutrient balance cannot be forgotten. Drugs commonly prescribed in kidney patients, like NSAIDs, Proton-pump inhibitors (PPI) or steroids, may also contribute to leaky gut. Changes in digestion due to some of these medications also contribute to altered nutrient absorption, including malabsorption of macronutrients, as well as micronutrients needed as cofactors in many biochemical reactions in the body.
Nutrients
The gut also can also serve as a source of increased oxidative stress, contributing to increased systemic inflammation that accelerates CKD and its comorbid conditions, including cardiovascular disease. Research suggests that one mechanism by which toxins generated by bacterial load speed up CKD progression is by altering metabolism and absorption of normally occurring nutrients. This may indicate that nutrient repletion maybe a necessary step for successful outcomes with probiotic re-inoculation.
The Bottom Line on Gut Restoration and Progression of CKD
This is why the use of an individualized comprehensive gut restoration protocol, that steps outside of the conventional linear model and takes into account the various layers described above can slow the progression of kidney disease. One such approach can be summarized by the 5R program which we will explain in details in the upcoming blogs.
Kidney Stones: The Integrative Approach to Prevention and Management
This blog is the first in a series discussing our integrative approach to kidney stones prevention and management.
Kidney stone, also called nephrolithiasis or urolithiasis, is a complex disease influenced by multiple factors including genetic and environmental factors. Stones are often painful, and left unaddressed can lead to more serious conditions such as obstruction of the urinary tract and permanent damage to the kidneys.
By Majd Isreb, MD, FACP, FASN, IFMCP
The frequency of kidney stones has been on the rise in the United States (US) according to nationally published data. The National Health and Nutrition Examination Survey (NHANES) has analyzed the health and nutrition status of the general populations for the past 30 years. According to analyses from these publications, the lifetime chance for developing kidney stone in an adult (age 20-74) increased from 3.2% in the 1970’s to 5.2% in the 1980’s. Most recent survey using data between 2007 and 2010 showed that the lifetime chance for kidney stones in an adult is now up to 8.8%. That’s almost a three-fold increase over three decades.
In addition to the inconvenience and pain associated with kidney stones, they also pose a significant healthcare burden and cost. Patients with kidney stones are likely to present to the emergency department and are often hospitalized for an average of 2-3 days. If patients cannot pass them, they may require surgical intervention. In 2000, the total costs for caring for patients with kidney stones in the US. was estimated at $2.1 billion. Furthermore, it is estimated that the cost of care will rise by $1.24 billion per year by 2030.
Kidney Stones: Types and Formation
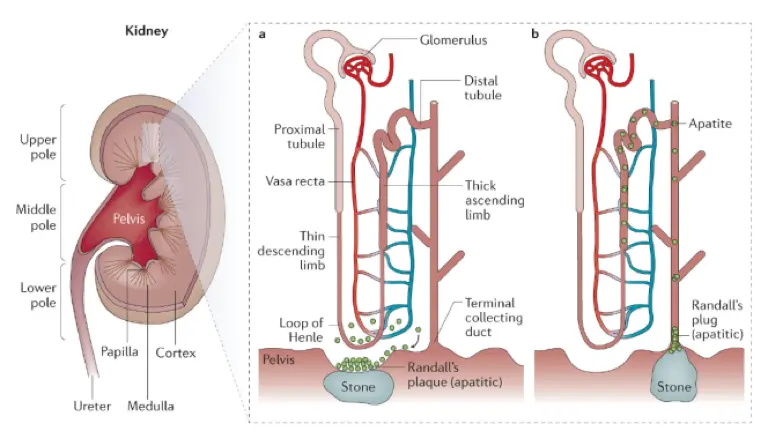
There are five major types of kidney stones: calcium oxalate, calcium phosphate, uric acid, struvite (magnesium ammonium phosphate), and cystine. Calcium oxalate is by far the most common, comprising approximately 75% of kidney stones.
Calcium oxalate and calcium phosphate stones
Calcium stones are the most common type of kidney stones. They are composed of either calcium oxalate or calcium phosphate compounds. They are formed when calcium binds to oxalate (or phosphate) in the urine. On the other hand, dietary calcium can bind oxalate in the intestine and prevents its absorption through the gut, so there is less in the urine to form stones.
Oxalates are compounds found naturally in certain foods (nuts, spinach, potatoes, tea, and chocolate). In those prone to calcium oxalate formation, eating high amounts of foods rich in oxalates can increase the amount of oxalate in the urine and increase risk of stone formation.
Calcium phosphate stones are less common than calcium oxalate stones. Causes include hyperparathyroidism (when the body produces too much parathyroid hormone), renal tubular acidosis (a kidney condition that causes a buildup of acid in the body), and urinary tract infections. It is important to understand if one of these conditions is behind the formation of calcium phosphate stones.
Inadequate hydration is a major risk factor for these types of stones. Certain medications can also reduce risk of stone formation, including thiazide diuretics (for example, hydrochlorothiazide) reduce calcium levels in the urine available to form stones. Potassium citrate binds to calcium, preventing it from binding to oxalate and phosphate to form stones.
Uric acid stones
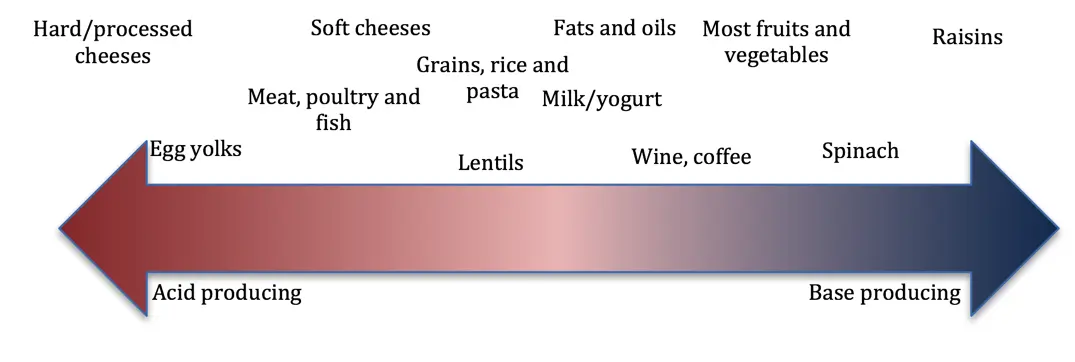
Uric acid stones generally form when urine is too acidic, causing otherwise normal levels of uric acid to dissolve into the urine where it may crystallize forming stones. Therefore, by alkalizing the pH of the urine, we can prevent crystal formation.
In these cases, potassium citrate is the most common medication used to manage uric acid stone formation. Another medication, sodium bicarbonate can also be used to alkalinize the urine.
However, in some individuals, consumption of too much animal protein can actually increase the production of uric acid. In these cases, dietary restriction of animal protein might be necessary to manage excessive uric acid production. Use of allopurinol, a medication that prevents uric acid formation from precursors xanthine and hypoxanthine may also be indicated.
Struvite stones
Struvite stones are composed of magnesium ammonium phosphate, and unlike other stones, form in alkaline urine. Most commonly, these types of stones form due to a bacterial infection that raises the urine pH to alkaline levels. To dissolve these stones, acetohydroxamic acid (AHA) is used to reduce urine pH and ammonia.
Cystine stones (least common kidney stone type)
Cystine stone formation (also called cystinuria) is a relatively uncommon type of stone and the result of a genetic condition. As a result, urinary elevations of the amino acid cystine result in stone formation. Cystine stones can often be managed by improving hydration and maintaining alkaline urinary pH through diet and medication.
The Integrative Approach to Kidney Stones Treatment
Conventionally, the treatment approach may include a multi-pronged approach and may include medication, dietary and lifestyle, surgical removal, and using ultrasonic waves to break up stones.
There are a few conventional dietary guidelines, but guidelines tend to focus too far downstream, on stone composition, not on the underlying pathology and across risk factors upstream to prevent formation. By understanding the pathology and risk factors involved, we can better understand why some people develop stones, while others do not and effectively reduce the incidence of urolithiasis.
Socioeconomic and Environmental factors that impact the development of kidney stones
Studies of the distribution of kidney stones in the US suggest that geography is an important consideration in risk. For example, individuals who live in southern states, are more likely to have kidney stones than those who live in the North. In fact, inhabitants of the Southeast are nearly twice as likely to have a history of kidney stones as compared to those living in the Northwest. This has earned North Carolina, South Carolina, Georgia, Alabama, Mississippi, and Tennessee the nickname “the stone belt.”
Incidentally, these states also lead the nation in obesity and the incidence of diabetes. Inhabitants of these states are also more likely to consume a Standard American Diet (SAD) increasing the risk of pH imbalance that can lead to kidney disease and risk of stone formation.
Another factor to consider is climate. Higher ambient temperature and sunlight index are associated with a higher risk for kidney stones. Also, the incidence of kidney stones is higher in the summer than in the winter. This may in part explain why inhabitants of the warmer states in “the stone belt” have such a high incidence of kidney stone formation.
Why is this significant? Understanding this disproportionate distribution can help us understand the complexity of kidney stone pathology to better build a better approach. Our integrative approach to prevention and treatment should be sensitive to underlying socioeconomic and environmental contributions as well as factors that reduced access to adequate healthcare and good nutrition.
Impact of Diet on Kidney Stones
When the subject of urolithiasis and diet comes up, recommendations about the intake of calcium, oxalates, and hydration cannot be avoided. However, the reality is that we should be looking at broader considerations when it comes to the integrative approach to kidney stone prevention.
Consumption of the standard American diet (SAD) seems to increase the risk of kidney stone formation. SAD includes the consumption of sugary beverages and soda, as well as elevated intake of processed foods and animal protein. Interestingly, eating more fresh produce is protective. This is associated with the nutritional benefit, including (but not limited to) foods rich in potassium magnesium, and fiber. Furthermore, taking vitamin D, and good hydration risk were reversed.
This topic deserves a deeper dive, and we do so in another blog [found here].
Genetics and Kidney Stones
There seems to be a familial link when it comes to the development of kidney stones. Recently, genome-wide association studies uncovered several genetic sequence variants (SNPs) that lead to an increased risk of kidney stone development. Single nucleotide polymorphisms (SNPs) have been associated with kidney stones including those found in CLDN14, ALPL, SLC34A1, CASR, VDR, OPN, and TRPV5.
These genes play a role in the way the kidney handles certain vitamins and minerals including vitamin D, calcium, and phosphate. Imbalances of these nutrients are involved in the pathophysiology of stone formation, therefore stands to reason that genetic variations that result in mishandling will increase the risk. There is still a lot more to learn about the contribution of genetic factors to stone formation. However, what we do know is that we can modulate these risks through environmental and dietary modification.
Microbiome and Kidney Stones
Balance of the gut bacteria also plays an important role in causing or preventing kidney stones. The most studied organism is Oxalobacter formigenes, which has been found to be protective when present in adequate quantities as part of the GI microflora. This bacterium degrades oxalate in the gut decreasing its absorption and excretion in the urine.
In addition, dysbiosis in general is linked to kidney stone formation in those people prone to stone formation due to genetic or environmental factors. Therefore (and unsurprisingly), gut health is an important consideration when addressing kidney stones. Antibiotics which negatively alter the gut microbiome, are linked to higher rates of kidney stones.
More on the contribution of the microbiome on kidney stone formation will be the topic of another blog on the gut-kidney access here.
The Bottom Line
Although genetic factors may impact risk of kidney stone formation, environmental, dietary, as well as factors affecting the integrity of the gut microbiome play a large role in turning on those genes and impacting stone formation. Therefore, the integrative approach to addressing kidney stones must account for a combination of all these factors and practitioners should formulate and personalized approach that modifies relevant lifestyle factors.
This blog was written with contributions from Lara Zakaria, RPh MS CNS CDN IFMCP
Sleep and Kidney Health
Many Americans report difficulties with sleep. Do any of these sound familiar?
- Regularly getting less than 7-8 hours of sleep per night
- Waking up in the morning not feeling fully well rested most mornings
- Regularly have trouble falling asleep
- Wake up frequently once or more during the night and can’t fall back asleep
- Wake up earlier than intended regularly
- Snore or told you have sleep apnea
- Suffer from daytime sleepiness
If these resonate with you, your sleep quality may be negatively impacting your kidney health. Research has uncovered a strong connection between sleep for general well-being as well as an important key for kidney health.
The Scale of the Problem
Sleep disorders are very common in patient with chronic kidney disease (CKD). Because sleep symptoms can be subjective, it’s difficult to nail down the exact prevalence. Studies report that the prevalence of sleep disorders in kidney patients ranges between 31-57% depending on published studies. Although it appears that there is no association between the severity of sleep disorders and the stage of kidney disease, those who have kidney failure and are on dialysis are more likely to have problem with sleep than other CKD patients.
When you look at specific sleep disorders such as obstructive sleep apnea (OSA) or restless leg syndrome (RLS) you will find that these are very common in patients with CKD. In fact, studies that measured the frequency of sleep apnea in CKD patients reported results as high as 94%.
Join us to end the kidney disease epidemic
Why Adequate Sleep is Important
Insomnia is the most common recognized sleep disorder. It is defined as the subjective complaint of difficulty in initiating or maintaining sleep for at least three times per week for a duration of four weeks or more to a degree that daytime functioning is impaired.
Sleep problems can lead to a decreased performance at work or school. They may slow reaction time increasing the risk for motor vehicle accidents. They have also been linked to depression, anxiety and substance abuse. Sleep disorders have been associated with increased risk for diabetes, high blood pressure and heart disease.
When thinking about sleep health, it is important to think about these following qualities:
- Sleep duration (the total amount of sleep in 24 hours)
- Sleep continuity or efficiency (the ease of falling asleep, staying asleep, and returning to sleep if woken during the night)
- Timing (What time are you sleeping)
- Alertness/sleepiness (the ability to maintain attentive wakefulness throughout the day)
- Satisfaction/quality (the subjective feeling of “good” or “poor” sleep)
A sleep questionnaire can be very helpful in identifying those who have sleep issues and need for intervention. Try our sleep questionnaire here if you’d like to understand your sleep quality better.
Disrupters of Sleep
Many factors can lead to sleep disruption. These factors include toxin exposure, genetic risk, nutritional deficiencies, medications, and of course stress and anxiety.
Toxins
The hours we are sleeping are very important for nerve cells restoration and clean-up. In fact, studies have demonstrated that sleep promotes clearance of neurotoxic waste products that accumulate during waking hours.
The sleep-wake cycle is also important in liver detoxification of toxins and medications. But most importantly, toxins such as arsenic, pesticides, phthalates, polyfluoroalkyl compounds (PFAs) have been specifically linked to sleep troubles in one of the largest studies conducted in the US. Higher levels of urinary arsenic were found to be associated with leg jerks. Pesticides were associated with increased leg cramps during sleep. PFAs not only can directly cause kidney damage, they also disrupt sleep due to increased leg jerks during the night.
Nutrient Deficiencies
There are several nutrients hat are implicated in disrupting sleep:
- Vitamin D , a hormone that interacts with many cellular receptors including those in the gut, bone, breast, prostate, brain, skeletal muscle and the immune system has been found to play an important role in the time to fall asleep.
- Maintaining good blood sugar balance by reducing excessive carbohydrates, and eating foods rich in fiber, healthy fats, and protein is also important for getting good quality sleep.
- Deficiencies of tryptophan, an amino acid precursor of serotonin and melatonin, or B6 which is needed to regulate sleep hormones may also impact sleep.
- Many micronutrient deficiencies have been associated with sleep disturbances, with the strongest link is found between magnesium and zinc deficiency.
Medications and alcohol
Many prescription drugs have been found to interfere with sleep. Medications such as antidepressants, asthma and blood pressure medications are the most common offenders. In addition, many over-the-counter medications such as pain medications, allergy, and cold medication and weight-loss products can contain caffeine or other stimulants that can disrupt sleep.
Even sleep medications, which are commonly used to treat insomnia may cause sleep disturbances. This is because they do not allow those who take them to fall into the normal, deep sleep pattern that results in restorative sleep. Alcohol may make you sleepy, however studies show that regular consumption of alcohol is also disruptive to healthy sleep.
Biological factors
As you may expect, stress has been documented to lead to poor sleep quality. This association is maybe mediated by the hormones that drive up activity of the hypothalamus-pituitary-adrenal (HPA) Axis, driving the fight-or-flight-response. Multiple hormones have been found to affects the sleep cycle, particularly cortisol for example. Cortisol is a driver of inflammation and can contribute to insulin resistance which leads to blood sugar imbalances, which we’ve already learned affect sleep quality.
Lastly, it appears that our genes play an important factor in setting our internal clock. Genes our sleep-wake patterns that can influence our physiology, our cyrcadan rhythm, we utilize nutrients, and handle toxins and medication – all the factors that impact sleep. The field of chronotherapeutics which studies these associations is still in its infancy but we will learn more about it in the near future.
Impact of Poor Sleep on Kidney Health
Poor sleep can impact the kidneys in two ways: directly and indirectly.
Indirectly, the factors we described above may lead to insufficient sleep, poor quality of sleep and apnea can lead to elevated blood pressure (hypertension) or make it more difficult to control. There is also a strong body of evidence linking blood sugar (glucose) metabolism with sleep quality and quantity. Fragmented sleep has been associated with increased insulin resistance and metabolic disease. Finally, fragmented sleep has been shown to impact the hormonal control of satiety and hunger leading to excessive eating and obesity. It is well established that diabetes, hypertension and obesity are associated with linked to the development and progression of CKD.
Directly, sleep can be a key regulator of kidney function. During sleep, sympathetic activity (fight-or-flight) decreases, and parasympathetic activity increases leading to a drop in blood pressure providing a positive benefit on the circulation in the kidneys. Patients with sleep disorders, especially those with sleep apnea may lose this drop in blood pressure because their parasympathetic system doesn’t kick on.
Shift work and irregular sleep timing and poor quality has also been found to affect the regulation of the renin-angiotensin-aldosterone system (RAAS). RAAS plays an important role in the development and progression of kidney disease. Last but not least, obstructive sleep apnea is associated with increased inflammation and oxidative stress which leads to kidney damage.
Impact of Kidney Disease on Sleep
CKD itself can be a risk factor for sleep disorders. Melatonin, which is a hormone secreted by the pineal gland, is responsible for the sleep-wake cycle usually increase during the night to induce sleep. In kidney patients, this natural rhythm seems to be blunted. CKD can lead to a short, fragmented sleep and difficulty falling asleep. In addition, CKD has been associated with OSA, restless leg syndrome (RLS), and increased leg cramps.
The Bottom Line
Sleep disorders are common in kidney disease and they have been associated with the development and progression of CKD. They also can lead to inflammation, diabetes, hypertension and obesity. Multiple factors plan a role in the development of sleep disorders. It is important to work with an Integrative or Functional medicine provider to evaluate your sleep and kidney health.
Lifestyle Modifications for Polycystic Kidney Disease
The phrase “our genes are not our destiny”, describes how nutrition and lifestyle factors can have a positive impact on genetic expression for certain diseases. This field of study is called epigenetics. Recently, researchers have found evidence that diet and lifestyle modifications can impact polycystic kidney disease (PKD).
What is Polycystic Kidney Disease?
PKD is a group of two genetic disorders that affects the kidneys and cause the formation of multiple fluid-filled cysts of various sizes. As these cysts grow, they squeeze and destroy normal kidney tissue, eventually leading to loss of kidney function. PKD is a Mendelian inherited disease, meaning it’s passed down genetically like eye or hair color. It said to be autosomal-dominant, slowly progressive disease affecting 50% of offspring, or autosomal-recessive which affect 25% of offspring of affected individuals but is usually rapidly progressive in childhood.
How does PKD develop?
Cysts that form in PKD usually occur when the cells lining the tubules of the kidney end up proliferating causing outpouchings (bulgings) that eventually separate into cysts. These cysts end up growing, transporting fluid across their lining into their lumen forming a fluid-filled sack or balloon. So, when we think about PKD, we should remember two processes: cell proliferation (cell growth) and fluid secretion into the cysts.

In this blog, we focus on factors that affect fluid secretion into the cysts. This process is mediated by a cellular messenger called cyclic adenosine monophosphate, or cAMP, which is activated by the actions of anti-diuretic hormone (ADH). It should be noted that it can also be activated by other hormones such as the parathyroid hormone, prostaglandin E2, epinephrine, among others, however we will focus on ADH.
What is ADH?
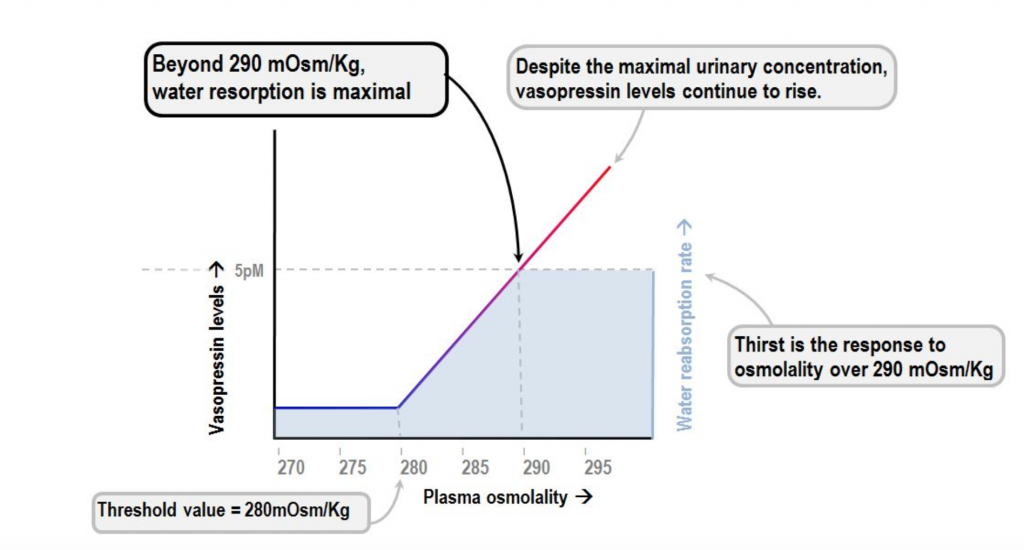
ADH (also known as vasopressin) is a hormone produced by the brain and excreted by the pituitary gland. The purpose of ADH is to tightly regulate water balance and osmotic pressure. The presence of this hormone is crucial for survival, it is what gives humans and other land-dwelling animals the ability to survive out of the water.
When the body’s receptor’s sense that there’s an accumulation of certain solutes such as sodium, a chain of events occurs to preserve water (fluid retention). The chain of events is triggered by an increase in ADH. The kidneys have receptors that respond to ADH by formation of cAMP which leads to increase water retention.
Sodium concentration in the blood is reflective of the amount of water in our body. High sodium (and osmolality) levels stimulate the production of ADH and preservation of water. Low sodium levels inhibit the production of ADH stimulating water release. In fact, at a certain low level of sodium concentration and osmolality ADH excretion will be shut off completely.
The role of ADH in PKD
ADH was found in several studies to promote cyst growth by stimulating the production of cAMP. Drinking large amounts of water to a degree that the urine osmolality is lower than serum osmolality leads to a significant decrease in the progression of PKD. Cyst growth was inhibited in both forms of PKD by administering ADH receptor blockers. In addition to dehydration, other factors that lead to the production of ADH include stress, pain, and surgery. These factors, therefore, can indirectly lead to cyst growth and further decline in kidney function.
What can you do if you have PKD?
The integrative approach to PKD focuses on drinking adequate amounts of filtered water. In general, we recommend that kidney patients who have no significant volume overload problems drink half of their body weight in ounces of water daily. So, for example if you weigh 190 lbs., aim to drink at least 95 oz of water daily. Patients with PKD should aim to have their water intake at the highest end and restrict their salt intake to decrease serum osmolality and ADH excretion.
Yoga and stress reduction techniques, such as meditation or breathing exercises, can also play an important role in inhibiting ADH excretion and other hormones that can activate cAMP such as epinephrine.
In addition, assuring adequate intake of high-quality vitamin D will help inhibit Parathyroid hormone (PTH - another hormone that influences cAMP production) and limit cyst growth.
Finally, there are foods and beverages that can lead to accumulation of cAMP by inhibiting an enzyme called phosphodiesterase (PDE). In certain conditions such as heart disease, some of these same foods may benefit, however they should be avoided by PKD patients to preserve PDE function and minimize cAMP. These include methylxanthines such as caffeine, found in coffee, mate, tea, and cacao. In addition, catechins found in green tea, and flavonoids found in red wine should be avoided. Some recent laboratory studies suggest that extracts of artichoke and ginger might also be contraindicated in PKD, but more research should be done to draw any conclusive outcomes.
As always, we recommend that you discuss these lifestyle recommendations for polycystic kidney disease with your provider or nephrologist since every kidney patient is unique.
Arsenic and Kidney Health
You might be surprised to learn that arsenic is micromineral naturally found in our food and in the soil. However, in higher amounts, arsenic is a toxic heavy metal. Even though arsenic poisoning is lethal, it’s important to realize that lower-level chronic exposure to arsenic can negatively impact kidney health. In this blog, we will discuss arsenic and kidney health
By Majd Isreb, MD, FACP, FASN, IFMCP
Arsenic and Kidney Health
Exposure to Arsenic has been associated with increased risk for cancer, and it is also linked to kidney disease. In this blog, we discuss the sources of arsenic exposure, how our body handles arsenic, its effects on the kidneys, and the genetic and epigenetics of arsenic.
Sources of exposure
Drinking water appears to be the greatest source of arsenic exposure worldwide. In addition, exposure from ingested foods comes from food crops grown in arsenic-contaminated soil or irrigated with arsenic-contaminated water. Even if the water you drink is filtered and does not have arsenic, it is possible that the food that you eat is contaminated with arsenic, particularly rice. Arsenic is also found in high concentrations in cigarettes.
Found naturally in the environment, arsenic is mobile and cannot be destroyed. When arsenic compounds interact with oxygen or other molecules or bacteria it changes into different forms that can dissolve in water. Cosmetics and personal care products are another often overlooked major source of exposure.
How our body detoxifies arsenic
After ingestion or exposure, arsenic is metabolized and detoxified by a process called methylation. An enzyme called arsenic methyltransferase (AS3MT) adds a methyl groups to arsenic compounds to help neutralize it and eliminate it. This enzyme has been detected in human liver, kidney, bladder, heart, lung, testes and adrenal glands. Glutathione, known as the master antioxidant also plays a key role in arsenic detoxification.
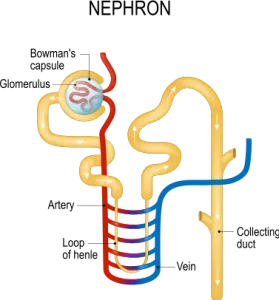
The various arsenic forms bind to glutathione. The compound arsenic-glutathione can easily exit the cells and circulate in the blood. The glutathione-bound arsenic compound can be excreted by the kidneys or in the bile. The kidneys are the major site of elimination of arsenic and they are, therefore, highly exposed to it. Arsenic can also accumulate in the kidneys. Many transporters have been implicated in arsenic excretion in the tubules portion of the filtering units of the kidneys (the nephron). Inhibition or genetic changes in these transporters can lead to enhanced arsenic toxicity.
Interestingly, the water channel, aquaporin 3, has been found to increase the uptake of arsenic in the kidneys. These channels play a crucial role in water handling by the kidneys. They help our body preserve water. Increasing water intake decrease the incorporation of these channels to the cells of the tubules in the kidneys. Therefore, increasing filtered water intake can help decreasing the kidney exposure to arsenic.
Finally, a transporter called multidrug resistance transport protein 2 (MRP2) transport arsenic-glutathione complexes and excrete them out of kidney cells into the urine. Studies show that the increase in the production of glutathione and MRP-2 increase the body’s ability to detoxify arsenic. Selenium is important for this process and selenium deficiency can enhance arsenic toxicity.
Arsenic effects on the kidneys
Acute exposure of the kidneys to toxic levels of arsenic leads to inflammation in the tubules of the kidneys. This can lead to protein loss in the urine. It can also lead to elevated calcium levels in the blood. Arsenic exposure activates cell growth can lead to cancer. Arsenic can also lead to oxidative stress and cellular injury. Arjunolic acid (an anti-oxidant which is present in fruit specially guava) was found to decrease the toxicity of the arsenic on the kidneys. Zinc is also protective. Chronic exposure can lead to high blood pressure, protein loss in the urine, and chronic kidney disease.
Join us to end the kidney disease epidemic
Genetics and Genomics Impact on Arsenic Toxicity
You can now see that various processes are involved in the handling of arsenic by the body from detoxification to transport to elimination. Genetic variants of any of these steps can either be protective or can increase arsenic toxicity. For example, a single nucleotide polymorphism (SNP), or genetic mutation, that increases glutathione production can be protective since glutathione helps to eliminate arsenic safely. However, a SNP in the gene that codes for MRP2 may potentially decrease arsenic excretion leading to increased toxicity.
Arsenic accumulation and toxicity can lead to oxidative DNA damage. It also inhibits DNA repair. This explains the increased risk for cancer after exposure to arsenic. Chronic exposure to even small concentrations of arsenic can have synergistic effect on other factors that can cause cancer such as UV light and smoking.
Arsenic exposure can cause indirect DNA damage through processes of DNA methylation and histone acetylation. These altered epigenetic expressions have been linked to kidney inflammation and damage. More recently, it was noted that long term exposure to low level of arsenic (below what is considered safe by the EPA guidelines) were associated with epigenetic changes that caused kidney scarring.
[bctt tweet=" it was noted that long term exposure to low level of arsenic (below what is considered safe by the EPA guidelines) were associated with epigenetic changes that caused kidney scarring." username="inkidney"]
The Bottom Line
Most arsenic exposure is due to drinking and showering with unfiltered water. Arsenic exposure leads to extensive cellular damage which increases the risk for cancer and kidney disease. Nutritional deficiencies can enhance its toxicity, but you can take steps to reduce your risks.
- Choose a home filtration device that effectively removes heavy metals, including arsenic. Not all consumer filters will remove arsenic, consider upgrading to Clearly filtered, Multipure and Aquasauna water filter systems.
- Always choose organic produce whenever possible, However, when it comes to rice and teas – organic isn’t enough. Look for products that screen for arsenic levels.
- Antioxidants are protective against arsenic damage, so a diet high in antioxidants like citrus fruit, green tea, blueberries, and dark chocolate will be naturally protective. Enhance detoxification by eating dark leafy greens, cruciferous veggies (like broccoli, kale, and cabbage), and sulforaphane-rich foods like garlic and onion contain high concentrations of nutrients that increase our detoxification capacity.
- Load up on fiber and mineral-rich root veggies, which help trap and eliminate toxins through your digestive system.
- There are several lifestyle modifications that can support improved detoxification, including Epsom-salt baths, sauna, lymphatic massage, sweat-inducing workouts, and dry brushing.
- If like many Americans you’re not having consistent daily bowel movements, you’re not adequately detoxifying and should consider working with a healthcare provider to address your digestion and motility.
Foot Note:
There are different forms of arsenic compounds. There are organic and inorganic forms with different oxidation states. The inorganic forms are easier to absorb by the gut and, therefore, are more hazardous. The most commonly ingested inorganic compounds are the trivalent and the pentavalent forms (depending on the level of oxidation). The trivalent forms appear to be the most toxic.
Vitamin D and Kidney Health
Kidney disease (KD) is associated with changes in bone health and mineral balance. Vitamin D is a fat-soluble vitamin that is essential for life and is crucial for calcium balance and bone health. The kidneys are central in the activation of vitamin D and play a key role in regulating circulating levels. In this blog, we will discuss the relationship between vitamin D and kidney health.
Vitamin D as a hormone
Vitamin D is a term used to describe a family of compounds derived from cholesterol. There are two major forms to keep in mind. Vitamin D2, or ergocalciferol, is mostly found in plants, and D3, or cholecalciferol, the form found in animal sources and produced naturally in our skin when we’re exposed to the sun. It is a “conditionally essential vitamin” because we need cholesterol precursors to produce it, but we can also consume it with food.
Vitamin D is not only a vitamin, but it’s also considered a hormone because of its direct interaction with cell receptors in the body. We rely on our “factory” in the skin to make Vitamin D when we are exposed to the sun. The UV rays stimulate the conversion of precursors derived from cholesterol into active vitamin D3. Like other hormones derived from cholesterol, such as estrogen and testosterone, not eating or producing enough cholesterol can contribute to a deficiency of vitamin D.
Foods that contain vitamin D
We typically think of vitamin D as “the sunshine vitamin.” However, various factors impact the ability of the body to produce adequate active vitamin D3, including average daily sun exposure, geographic location, skin color, and genetic variations that impact “the vitamin D factory.” Luckily, there are other options for meeting our needs, including consuming vitamin D-rich foods as well as taking a high-quality supplement.
Foods that naturally contain the active form of vitamin D3 include wild salmon, herring, sardines, cod liver oil, tuna, oysters, shrimp, and egg yolks. Surprisingly, mushrooms are the only plant-based source of natural vitamin D2. However, you can actually significantly increase the naturally occurring amount of D3 by laying mushrooms in the sun (learn more here). Since Vitamin D is a fat-soluble vitamin, absorption is best when eaten with a healthy fat or food naturally containing fat.
Many people associate milk with food sources of Vitamin D. However, milk is not naturally a good source of vitamin D. Beverage companies fortify milk and other drinks (like orange juice and nut milk products) and market them as “great sources of vitamin D” – in other words, vitamin D is artificially added in during the manufacturing process.
Vitamin D receptors
Vitamin D is most commonly associated with bone health because it’s necessary for the bone formation process, along with vitamins K, calcium, magnesium, and phosphorous. However, it’s vital beyond bone health. Receptors for vitamin D have been identified in almost all organs in the body. These genes are responsible for the calcium and phosphate balance, immune response, and cell growth and differentiation. The presence of vitamin D receptors in the blood vessels also indicates that vitamin D plays an important role in maintaining heart health.
Activation of Vitamin D by the kidneys (and the liver)
Vitamin D that is produced by our skin or consumed is transported to the liver as a “prohormone” by a protein called Vitamin D Binding Protein (DBP). In the liver, this precursory form is converted by an enzyme called 25-hydroxylase (or CYP2R1). The end-product is called 25-hydroxyvitamin D, abbreviated 25-(OH)D, and is the main circulating form of vitamin D.
Once produced, 25-(OH)D is eventually transported to the kidneys, where another (-OH) group is added. The result is the active hormone called 1,25-Dihydroxyvitamin D (or 1,25-Dihydroxycalceferol, abbreviated 1,25-(OH)2D).
Vitamin D receptors are mainly activated by 1,25 (OH)2D. However, some cells may have a modest capacity to activate vitamin D locally. In addition, at a very high concentration, the less active 25 (OH)D can bind with vitamin D receptors,
Genetic variations in the activating enzymes involved in this multi-step process are well documented and influence the individual ability to activate the prohormone into the needed active product. This is why checking both the 25-(OH)D and the 1,25 (OH)2D levels may give us a better idea of the true level of bioavailable vitamin D.
Vitamin D in Kidney Disease
In KD, the gradual loss of functional kidney tissue responsible for the activation of vitamin D contributes to the deficiency of the active form 1,25 (OH)2D. Interestingly, more than 80% of KD patients also have a low level of the precursor form 25(OH)D when measured in the serum. Several factors have been implicated in the cause of this deficiency, including inadequate outdoor physical activity, inadequate dietary intake, genetic variations, and impaired retention of the filtered 25(OH)D by the kidneys. There is also evidence that the accumulation of waste products, a common effect of KD, can decrease the production of 25(OH)D by the liver.
Remember, vitamin D circulates in the blood bound to DBP. Without this protein to provide transportation, vitamin D precursors will not reach the liver for the step of activation to 25(OH)D. Since some KD causes urinary protein loss, low levels of DBP may contribute to low 25(OH)D in kidney patients. However, there’s conflicting evidence of the significance of this factor. There may be more to the story worth continued research, especially surrounding the genetic variations in the gene that codes for DBP and its subsequent effect on production and binding capacity.
We can’t talk about vitamin D and KD without talking about the parathyroid gland (not to be confused with the thyroid gland). The main function of the parathyroid gland is to maintain blood levels of circulating calcium, a mineral very important in heart and bone health, as well as normal muscle function.
As kidney function declines, phosphate accumulation indirectly contributes to a further reduction in vitamin D activation. These compounding factors promote the production of parathyroid hormone (PTH) by the parathyroid gland. KD). PTH maintains calcium by influencing absorption from the gut as well as increasing its reabsorption during kidney filtration.
When calcium levels in the blood drop too low and endanger cardiac function, it triggers PTH also to mobilize calcium from the bone storage into the blood to normalize circulating levels. This is the contributing mechanism that leads to a high bone turnover, weakened bones, and increased risk of fracture in kidney patients. In addition, vitamin D deficiency in kidney patients has been associated with muscle weakness, falls, insulin resistance, enlargement of heart muscles, blood vessel disease, and calcifications.
The target level of vitamin D in kidney disease patients
There is controversy surrounding the ideal target goal of 25 (OH)D and 1,25(OH2)D for kidney patients. Studies showed that the maximum benefit to decrease muscle weakness and fall risk in kidney patients is in the range between 24-44 ng/mL and that levels less than 15 ng/mL have been associated with increased risk for mortality and progression to dialysis in kidney patients. Conventionally, a target level of ~40 ng/mL but has been the standard of care. However, some practitioners argue that some patients may benefit from higher circulating levels.
Conventional and Integrative Approach to bone health in kidney disease
The conventional medicine approach to bone health in KD has been focused on correcting 1,25(OH)2D levels and decreasing PTH levels (though target levels in KD are unclear). Utilizing active vitamin D analogs has been linked to improved outcomes in KD and dialysis patients.
When addressing vitamin D’s impact on KD risk, we need to pay close attention to dietary factors that impact nutrient status (including calcium, magnesium, vitamin K, and other relevant nutrients), as well as digestive issues that may reduce nutrient absorption from food. In addition, the role of genetic and epigenetic modifications in coding for factors that impact vitamin D activation and vitamin D receptors might mean that simple recommendations to “get more sun” or supplement may not be adequate for some individuals and warrant a more personalized approach to optimize kidney health.
References:
1. Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281.
2. Townsend, K.; Evans, K.N.; Campbell, M.J.; Colston, K.W.; Adams, J.S.; Hewison, M. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment.
3. J. Steroid Biochem. Mol. Biol. 2005, 97, 103–109. Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010, 9, 709–715.
4. Eknoyan, G.; Levin, A.; Levin, N.W. Bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201.
5. Ishimura, E.; Nishizawa, Y.; Inaba, M.; Matsumoto, N.; Emoto, M.; Kawagishi, T.; Shoji, S.; Okuno, S.; Kim, M.; Miki, T.; et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25 dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999, 55, 1019–1027.
6. Nguyen-Yamamoto, L.; Karaplis, A.C.; St-Arnaud, R.; Goltzman, D. Fibroblast Growth Factor 23 Regulation by Systemic and Local Osteoblast-Synthesized 1,25 Dihydroxyvitamin D. J. Am. Soc. Nephrol. 2017, 28, 586–597.
7. Slatopolsky, E.;Weerts, C.; Thielan, J.; Horst, R.; Harter, H.; Martin, K.J. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J. Clin. Investig. 1984, 74, 2136–2143.
8. Kim, S.M.; Choi, H.J.; Lee, J.P.; Kim, D.K.; Oh, Y.K.; Kim, Y.S.; Lim, C.S. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J. Ren. Nutr. 2014, 24, 20–25.
9. Cankaya, E.; Bilen, Y.; Keles, M.; Uyanik, A.; Akbas, M.; Gungor, A.; Arslan, S.; Aydinli, B. Comparison of Serum Vitamin D Levels Among Patients With Chronic Kidney Disease, Patients in Dialysis, and Renal Transplant Patients. Transpl. Proc. 2015, 47, 1405–1407.
10. Kalousova, M.; Dusilova-Sulkova, S.; Zakiyanov, O.; Kostirova, M.; Safranek, R.; Tesar, V.; Zima, T. Vitamin D Binding Protein Is Not Involved in Vitamin D Deficiency in Patients with Chronic Kidney Disease. Biomed. Res. Int. 2015, 2015, 492365.
11. Garcia-Canton, C.; Bosch, E.; Ramirez, A.; Gonzalez, Y.; Auyanet, I.; Guerra, R.; Perez, M.A.; Fernandez, E.; Toledo, A.; Lago, M. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol. Dial. Transpl. 2010, 26, 2250–2256.
12. Namir, Y.; Cohen, M.J.; Haviv, Y.S.; Slotki, I.; Shavit, L. Vitamin D levels, vitamin D supplementation, and prognosis in patients with chronic kidney disease. Clin. Nephrol. 2016, 86, 165–174.
13. Kendrick, J.; Cheung, A.K.; Kaufman, J.S.; Greene, T.; Roberts,W.L.; Smits, G.; Chonchol, M. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am. J. Kidney Dis. 2012, 60, 567–575.
14. Yousefzadeh P, Shapses SA, Wang X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int J Endocrinol. 2014;2014:981581.
Feeding Gut Bacteria in Kidney Disease
Our gut is home to 100 trillion beneficial bacteria and what we eat has a tremendous impact on their composition. These bacteria constantly interact with the lining of the gut and their health is important for the maintenance of a healthy gut barrier as well as our overall health, especially in patients with kidney disease. In this blog, we will discuss feeding gut bacteria in kidney disease.
You can think of your gut’s bacterial ecosystem (or microbiota) as a society. Just like any society, there are good guys and bad guys, and we can measure such attributes as population and diversity. So, the three questions we ask when evaluating the microbiome are:
- Are there any bad bacteria that need to be evicted?
- What’s the bacterial population? Do you have enough inhabitants living in the gut?
- Is there enough bacterial diversity? Different bacteria have different jobs and contributions, so a broader diversity is an indicator for better health.
There are multiple factors that affect the answers to those questions, and here we’ll focus on diet. Eating the right foods is possibly the most effective way to cultivate a robust microbiome and defend against “bad-guy overgrowth”.
[bctt tweet="Eating the right foods is possibly the most effective way to cultivate a robust microbiome and defend against “bad-guy overgrowth”" username="inkidney"]
Feeding Gut Bacteria in Kidney Disease
Probiotics and Prebiotics: What’s the difference?
Prebiotics is a term used to describe “bacteria food” needed to cultivate a healthy microbiome. Bacteria primarily feed on fiber found in vegetables, fruit, and whole grains (not processed grains like bread and pasta). In fact, in people eating the Standard American Diet (SAD), which is high in animal protein and fat and low in dietary fiber, we see microbiome quantity and diversity significantly reduced. This imbalance is thought to be one of the major contributors to the chronic disease epidemic in the US.
Probiotics are live bacteria (and sometimes yeast) that are usually consumed through traditionally fermented foods, like kefir, kombucha, and sauerkraut and other fermented veggies, and are good for optimal gut function. They can also be taken as a supplement, which have grown in popularity as more research has suggested a benefit of supporting healthy gut bacteria.
As it turns out, focusing only on probiotics has yielded mixed results in the research. That’s because bacterial levels in the gut are transient unless supported with long-term dietary changes that incorporate regular intake of prebiotic fiber. Therefore, we need to look at both pre- and probiotic intake to optimize the microbiome.
[bctt tweet=" It turns out, focusing only on probiotics has yielded mixed results in the research. That’s because bacterial levels in the gut are transient unless supported with long-term dietary changes that incorporate regular intake of prebiotic fiber" username="inkidney"]
Fiber, Fiber, and More Fiber
There are two kinds of fiber: soluble and insoluble.
Soluble fiber is the kind of food that sucks up water and becomes like a gel. This helps to move digested food through the gut. Insoluble fiber does not break down during digestion. It passes through the gut intact, forming a sort of net, binding digested food byproducts together to create firm stool.
Fiber intake of at least 30 g/day is associated with reducing the risk of heart disease, obesity, diabetes, and even certain kinds of cancers like colorectal cancer. These benefits are likely due to improved bowel movements, removal of toxins, and support of a healthy microbiome.
To preserve kidney function, animal protein sources should be reduced or restricted (more about that here). The research suggests, that plant-based protein sources might be ideal for kidney disease patients since they also double as sources of diverse dietary fiber.
Whole grains, legumes, nuts, and seeds make excellent sources of both fiber and protein. In addition, many vegetables and fruits have surprisingly significant amount of protein including avocado*, broccoli, cauliflower, mushrooms (especially Portobello and shitake), and seaweed.
Always keep in mind, though these foods may be generally healthy, in some patients with potassium restrictions, you need to consult with your renal nutritionist to personalize your diet.
Remember to always choose whole, fresh foods whenever possible and avoid processed foods that claim to have “added fiber”. They are usually packed full of fillers, sugars, grains, cereals and artificial ingredients.
[bctt tweet="Remember to always choose whole, fresh foods whenever possible and avoid processed foods that claim to have “added fiber”. They are usually packed full of fillers, sugars, grains, cereals and artificial ingredients" username="inkidney"]
The recommended daily fiber intake is somewhere between 30-40 grams of both soluble and insoluble fiber. The following options allow you to mix and match to achieve your daily fiber. Every kidney patient is different and needs an individualized approach; we’ve highlighted the foods within this list that are high in potassium with this symbol (*) and food high in phosphorus with this symbol (‡) so you can further customize to fit your needs:
- 1 cup of cooked oatmeal = 4 g
- 1/2 cup of raspberries = 4 g
- 1 cup of avocado*= 10 g
- 2 cups of romaine lettuce = 2.4 g
- Medium-sized banana*= 3.1 g
- 1 cup of carrots = 3.6 g
- 1 cup of beets*= 3.8 g
- 1 artichoke*= 10.3 g
- 1 cup of Brussels sprouts = 6.4 g
- 1 cup of kidney beans‡= 11.3 g
- 1 cup of cooked split peas*= 16.3 g
- 1 cup of cooked chickpeas = 12.5 g
- 1 cup of cooked quinoa*= 5.2 g
- 1 ounce of dried chia seeds‡= 10.6 g
- 1 cup of blueberries = 3.5 g
- 1 cup of strawberries = 3.3 g
- 1 cup broccoli = 3 g
- 2 carrots = 6.4 g
- 1 cup of brown rice = 3.5 g
- Half a cup of lentils‡*= 15.6 g
- Medium-sized boiled sweet potato*= 3.8
- 1 oz of almonds = 3.3 g
- 1 apple = 5 g
- 1 pear = 6 g
- 1 ounce of dark chocolate‡= 3.1 g
- 3 cups of popped popcorn = 3 g
[bctt tweet="The recommended daily fiber intake is somewhere between 30-40 grams of both soluble and insoluble fiber" username="inkidney"]
Cut out the sugar, You Are Sweet Enough Already
High-sugar diets can be a major disrupter of your gut microbiome, primarily because it feeds bad bacteria and yeast overgrowth. This is one of the proposed mechanisms contributing to metabolic diseases like diabetes and heart disease, two conditions associated with KD.
Should you take probiotics supplements?
Though supplementation may be very useful in some cases, the best long-term strategy is to increase the intake of per- and probiotics naturally through your diet. Traditionally fermented foods are a great source of naturally found probiotics, these include non-pasteurized traditionally-made kefir, sauerkraut, kimchi, miso, and pickled vegetables. However, for various reasons they may be restricted in some patients, so work with a nutritionist to implement the best strategy for gut bacteria balance – which may include a combination of food-based intervention and supplementation.
Other lifestyle factors that affect the microbiome
Interestingly, exercise may change the composition of the microbes in the gut for the better. Aim for at least 20 minutes of exercise daily and make it priority (you and your kidneys are worth it). In addition, stress can deplete the friendly flora and promote the growth bad bacteria. Although stress cannot always be avoided, find way to manage stress like breathing exercises and meditation, and do your best to avoid triggers.
It’s important to work with a physician and nutritionist familiar with an integrative approach to kidney disease. He/she can help you design a comprehensive plan that includes personalized dietary recommendations and supplements that promote healthy bacteria balance for optimal gut and kidney health.
The Age-Related Kidney Decline: Role of Nitric Oxide and Arginine
In the early 1950’s, researchers declared that age-related kidney decline reduced function at a rate of about 8% every 10 years after the age of 40. More recently, it was noted that the decline may begin as early as 20 years old. In this blog, we will discuss age-related kidney decline.
Each of us are born with a specific number of nephrons, the functional kidney units which does the job of filtering toxins out of our system. Depending on factors like diet, lifestyle factors, and genetics/epigenetics (to name a few), the rate of decline varies and results in different outcomes to each patient. So, is there anything we can do to reduce our risk of age-related kidney function decline to avoid dialysis?
What causes the Age-related kidney decline?
- Blood flow
Recent studies demonstrate that the blood flow to the kidneys decreases by approximately 10% every 10 years after age 40, affecting each nephron. In addition, the arteries of each nephron become less responsive to changes in blood pressure, increased pressure leads to damage and accelerated loss of kidney mass.
- Inflammation
Furthermore, as the lining of the filters inside the nephron become more permeable protein begin to seep through, contributing to increased inflammation. As time passes, inflammation leads to scarring of the filtration units. In fact, on average by the time an individual reaches the end of their 80’s, 30% of the nephrons are too damaged to be functional.
[bctt tweet="Inflammation leads to scarring of the filtration units. In fact, on average by the time an individual reaches the end of their 80’s, 30% of the nephrons are too damaged to be functional" username="inkidney"]
- Renin-Angiotensin System (RAS)
Factors that affects the blood flow to each nephron includes a system called the Renin-Angiotensin System (RAS). So that if the blood flow to the nephron declines, the nephron produces a chemical called Renin which promotes the production of another chemical called Angiotensin (Angiotensin I then Angiotensin II).
Angiotensin II increase the pressure inside the nephron and maintain adequate function by promoting “clamping” of some of the vessels in the nephron. With aging, production of Renin decreases. This results in a subsequent decline in the kidney’s ability to maintain good flow in response to changing blood pressure.
- Nitric Oxide production
Blood vessels are also controlled by Nitric oxide (NO2), another system that functions by expanding the blood vessels inside the nephron to help maintain good blood flow. This system also serves to protect the kidney from the formation of scar tissue.
Many factors help improving the production of NO2 while others lead to its breakdown. One example, are factors like genetics or availability of precursors that can influence the action of the enzyme responsible for making NO2, Nitric Oxide Synthetase (i.e. eNOS, iNOS, and nNOS). Another factor affecting the breakdown of Nitric Oxide are sex hormones. Estrogen, the dominant sex hormone in females, prevents the breakdown of NO2keeping its level high. Meanwhile, testosterone, the male dominant sex hormone, has the opposite effect of increasing its breakdown. This may in part explain why hypertension and kidney function decline is more pronounced in men than in women.
With aging, NO2 production declines and leads to the closing of the arteries in the kidney, sodium retention, and scar tissue formation. These factors make the aging kidneys more vulnerable to even minor changes in blood pressure, changes in hydration, and medication – in turn, making them more susceptible to acute kidney injury.
[bctt tweet="With aging, NO2 production declines and leads to the closing of the arteries in the kidney, sodium retention, and scar tissue formation" username="inkidney"]
What can be done to slow the rate of age-related kidney decline?
Conventional medicine focuses on using medications that block the RAS system such as Angiotensin Converting Enzyme (ACE) Inhibitors (i.e. enalapril, lisinopril) or Angiotensin II Receptor Blockers (ARBs) (i.e. losartan, irbesartan). There are a multitude of studies demonstrating their effectiveness in delaying the progression of various forms of kidney disease, including diabetic kidney disease, age-related kidney decline and others.
However, despite the delayed progression, patients continue to lose kidney function and end up requiring dialysis. Additionally, like all drugs, these medications come with side effects and risks*. Adverse effects include increased potassium retention, zinc depletion, and swelling of the throat, among other side effects. In fact, these medications have an established increased risk of acute kidney injury, particularly in elderly with kidney disease.
Arginine (or L-Arginine, we’ll use the terms interchangeably) is an amino acid that is essential for the body to produce NO2. It’s labeled as a conditionally essential amino acid because although the body can make it, production is dependent on factors including health and nutrition status. Levels tend to decline with age, therefore ensuring adequate intake through food sources or supplementation is important.
Arginine-rich foods include animal proteins, eggs fish, nuts (including walnuts, hazelnuts, pecans, peanuts, almonds, cashews, and Brazil nuts), seeds (especially pumpkin, also sesame and sunflower), oats, buckwheat, and cacao. Note, some of these foods are also high in phosphate which should be restricted in kidney patients even at early stages. (link-blog to write). With age and reduced gut function, absorption of nutrients from food may be diminished, so supplementation may be in order to prevent/slow age-related kidney decline. For kidney patients over 50-years-old, the recommendation* is to supplement 2 grams of L-Arginine three times a day to maintain good kidney circulation, stabilize function and prevent age-related kidney decline.
*Reminder: Always consult with your kidney doctor or primary care physician before taking any supplement, and never change or discontinue your medication without the instruction of your doctor.
References:
- Davies Df, Shock NW, Age Changes in glomerular filtration rate, effective plasma flow, and tubular excretory capacity in adult males. J Clin Ivest 1950; 29:496-507
- Llorens S, Fernandez AP, Nava E. Cardiovascular and renal alterations on the nitric oxide pathway in spontaneous hypertension and aging. Clin Hemorheol Microcirc 2007; 37:149-156
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Aging diminishes endothelium-dependent vasodilation and tetrahydrobiopterin content in rat skeletal muscles arterioles. J Physiol 2008; 586:1161-1168
- Auelo JG. Normotensive ischemic acute renal failure. N Engl J Med 2007; 357:797-805
- Ahmed SB, Fisher ND, Hollenberg NK. Gender and the renal nitric oxide synthase system in healthy humans. Clin J Am Soc Nephron 2007;2:926-931
IgA nephropathy: A target for Integrative approach
Immunoglobulin A (IgA) nephropathy is a prime example of the interplay of genetics, epigenetics, leaky gut, environment and diet affects and influence the development of kidney disease.
What is IgA?
Immunoglobulins are a large protein structure that is part of the adaptive immune system. There are various types, categorized by a letter and sometimes a number to identify the subclass, for example IgA1and IgA2. For the context of this discussion, we will the focus immunoglobulin A (IgA) without distinction to subclass.
IgA is found in the blood serum, lining of the respiratory (lungs) and digestive (gut) tracts. It is also found in saliva, tears, and breastmilk. Also called an antibody, the body makes IgA and other types of antibodies to help fight off invading pathogens (i.e. bacteria, viruses) and prevent disease. It is one of the first lines of defense against invading organisms from the environment, diet, and toxins.
What is IgA nephropathy?
IgA nephropathy is an autoimmune kidney disease. A trigger causes the immune system to produce abnormal IgA proteins that causes the immune defense to damage the kidney (nephrons). Abnormal IgA immune complex structures are formed, they circulate and filter through the kidney where they are “captured”. Unfortunately, these structures build-up in the kidneys, resulting in inflammation which damages kidney tissues.
What triggers an IgA immune response?
Though there’s some evidence that points to certain heavy metals (like cadmium) as a source of toxicity, the strongest evidence in the literature points to triggers can be classified into the following categories:
- Food sensitivities/allergies (i.e. gluten, soy, lectins/lipopolysaccharides)
- Microbial imbalance (known as dysbiosis) in the intestinal tract
Several studies have demonstrated an association between intake of gluten and lectins. One study demonstrated that patients with IgA nephropathy who followed a gluten-free diet for 6 months had a reduction in IgA levels. Studies on Mediterranean people eating a diet rich in gluten-containing foods published as far back as 1986 have suggested a dietary component to kidney disease. Soy is another example of a food antigen that has been associated with IgA nephropathy. However, heavy use of pesticides (glyphosate) in our food sources (especially gluten and soy) may play a role in food-related nephropathy. [bctt tweet="Heavy use of pesticides (glyphosate) in our food sources (especially gluten and soy) may play a role in food-related nephropathy" username="inkidney"]
In susceptible individuals, the intake of a dietary trigger leads to intestinal permeability (IP), also known as leaky gut. In fact, research dating back decades has supported a connection between the gut and IgA nephropathy, called the gut-kidney connection. One of the proposed mechanisms, is the development of microbial imbalance (dysbiosis) which leads to bacteria and food proteins “leaking” through the gut barrier, triggering IgA immune response damaging the kidney.
The role of dysbiosis
In the case of IP, the compromised lining of the gut (mucosa) allows abnormal microbes, endotoxins (toxins produced by microbes), or food particles and metabolites to enter the bloodstream.
Furthermore, studies have shown that patients with progressive IgA nephropathy tend to have low number of naturally occurring healthy bacteria in the gut (likeBifidobacterium species) and a high amount of unwanted, or pathogenic, bacteria (likeSterptococceae). Our gut lining cells have complex mechanisms to detect the type of microbes that are present in our gut. There is a consistent link between these mechanisms and the production of an abnormal IgA.
The genetic link to an abnormal IgA response?
Genetics likely also play a role in the risk of developing kidney disease. Researchers have identified genetic variances (also referred to as single nucleotide polymorphisms, or SNPs) linked to IgA nephropathy in a multinational study.
Most of the genes involved in regulating the response and immunity against gut microbes. Interestingly, many of these SNPs associated with IgA nephropathy risk are related to inflammatory bowel diseases (IBD) or with the maintenance of the intestinal barrier. These SNPs lead to abnormal trafficking of IgA and the formation of an abnormal IgA that triggers the body’s immune response.
The autoimmune response, putting it all together
Recall, the presence of abnormal IgA in the blood leads to the formation of protein complexes in response to neutralize the threat. Unfortunately, these proteins end up forming structures that are difficult for our body to eliminate. These complex structures with abnormal IgA end up depositing in the kidney, leading to local inflammation and loss of kidney function.
Now it should be clearer that IgA nephropathy is a classic example of how a combination of genetic, environmental factors, dietary and gut integrity interact to cause disease.
[bctt tweet="IgA nephropathy is a classic example of how a combination of genetic, environmental factors, dietary and gut integrity interact to cause disease" username="inkidney"]
Conventional medicine approach
The conventional approaches have failed to find a completely effective treatment for IgA nephropathy. Approaches include conservative control of blood pressure and blockage of the angiotensin-renin pathway, reduction of protein in the urine, and use of immunosuppressive therapy (including steroids and other medications), and fish oil.
More recently, a special kind of steroid that targets the terminal part of the gut (ileum and cecum) is being studied. The hope is that these special steroids will focus on one aspect of the process that leads to the formation of abnormal IgA which is decreasing the immune response in the gut.
Integrative medicine approach
A more integrative approach to IgA nephropathy would personalize treatment to take into account the impact of IP:
- Identifying the and eliminating the dietary or environmental triggers that contribute to leaky gut
- Assess and address dysbiosis
- Capitalize on epigenetic modifications to reduce the expression of SNPs contributing to IgA upregulation.
In combination with evidence-based conventional medicine, this approach targets various mechanisms and complications of the disease and improving outcomes.
Environmental Toxins and Kidney Health
On a busy weeknight, takeout and fast food are easy dinner solutions. But your family’s favorite to-go meal may come with a side of toxic fluorinated chemicals. These are called polyfluoroalkyl substances (PFAS) and they are a family of greaseproof, waterproof and non-stick industrial compounds. They are used in hundreds of consumer products, including ones that touch your food. PFAS is among a group of Persistent Organic Pollutants (POP) that can lead to kidney damage. In this blog, we will discuss these environmental toxins and kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Sources of Exposure
The impact of environmental exposure to these chemicals is increasingly recognized. In the past, the focus was on heavy metals such as lead and cadmium. The major risk to kidney health was job-related exposure. More recently, emerging research is proving that the general public is also at risk for exposure to a wide range of chemicals as a consequence of normal daily activities.
The activities that increase exposure to chemicals that negatively impact kidney health include:
- Dietary intake of food or drink, such as contamination of drinking water with these chemicals
- Domestic and commercial food preparation due to food additives
- Textile and household maintenance procedures as in preparing furniture with flame retardants
- Routine medical and dental care can be associated with exposure to toxic chemicals, for example, implants or dental fillings
The increase in environmental pollution, a result of accelerated urbanization worldwide, has become a global public health challenge.
Persistent Organic Pollutants (POP) and Kidney Health
Recently, POPs have been detected on military bases, as well as in public water supplies from industrial contamination and in agricultural and crop products. Exposure to POP has been linked to cancer including cancer of the kidney. There is also mounting evidence that POPs contribute to kidney disease. The kidneys are very sensitive organs. Studies demonstrated that the kidneys are the major site of elimination of these substances which makes them vulnerable to their negative effects.
In fact, studies point to PFAS as contributing directly to the worsening of kidney function. They can also indirectly lead to changes in human metabolism, which can lead to kidney disease.
Toxicology studies show that the tubules of the kidneys are the most affected (see our blog about the nephron). When part of the kidneys is affected, their function is reduced. This leads to a further buildup of these chemicals increasing their toxicity on the body and the kidneys. This creates a vicious cycle that amplifies the effect of toxic exposure. What is most concerning is that children and adolescents are and will be more exposed to these substances throughout their life span.
The Role of Genetics
Our bodies detoxify foreign chemicals in the liver via specific processes and they are eliminated in the kidney by other routes. The failure of these processes of detoxification and elimination may lead to greater toxicity of POPs. Genetics, epigenetics, or minor changes in the detoxification processes can have a significant impact on kidney health. Therefore, a normal exposure level for one person may be toxic for another, especially in the long term. Therefore, checking for genetic abnormalities is a crucial element in identifying the risk of exposure.
Heavy Metals
For years, kidney doctors (nephrologists) have known that heavy metals can impact kidney health. The most well-known of these include Arsenic, Lead, and Cadmium.
Arsenic is used in the pressure treatment of wood. It can lead to structural changes in the kidneys which can lead to kidney disease. Contamination of drinking water, soil, and rice with arsenic has been linked to hypertension and kidney injury leading to kidney disease.
Cadmium is used in batteries, pigments, coatings, and plastics. It has also been used in phosphate fertilizers and can lead to soil and water contamination with subsequent accumulation in plants, particularly root vegetables (carrots, etc.) It can also be found in tobacco cigarettes and inhaled in second-hand smoke. In fact, it is estimated that individuals who smoke one pack of cigarettes each day will absorb 1-2 mcg of cadmium daily. Cadmium exposure leads to the development of diabetes, decreased renal function, increased urine output, and loss of some minerals from the kidneys. It appears that vitamin D and Zinc can protect the kidneys from cadmium.
Lead is used in welding, batteries, and in the production of pottery, glazing of ceramic pots, and water pipes (which can lead to contamination of community water as happened in Flint, MI). Many children are exposed to lead by ingestion of contaminated soil or food and herbs grown in those soils. Sadly, as recently as 2017, a large population of adults and children in the United States have blood lead levels that are higher than what is considered safe. In addition to cognitive delays in children, relatively modest levels of Lead in the body can cause progressive kidney damage, elevated blood pressure, and gout.
Testing for Exposure
Unfortunately, after chronic exposure to these pollutants, our body adapts and stores them in tissues such as fat or bone. So, a simple blood test may not detect these substances. The best analysis to detect them is a provoked urine collection. This requires a physician familiar with carefully provoked urine testing to order a medication that encourages the release of these toxins from storage so that they can be collected in your urine. If you suspect you are exposed ask your Integrative or Functional Medicine nephrologist to test you.
References
- John W. Stanifer, et al. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health. Clin. J. Am. Soc. Nephrol. 2018. 13:1479-1492
- Sarah E. Orr, Christy C. Bridges. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017. 18, 1039
The Functional Kidney Mass: A risk factor for kidney disease
This blog is the beginning of a series of blogs about the risk factors for kidney disease. In this first blog, let’s start with talking about the functional kidney mass at birth and itsconnection of future kidney health. The development of the human kidney is generally completed by the 36th week of pregnancy in the unborn baby. Most of the filtering functional units (nephrons) of the kidneys are completed before the full term birth. These filtering units are called the nephrons.
Defining the Nephron: The basic filtering unit of the kidney
Each kidney contains several tiny filtering units called the nephrons. Each nephron includes two sections: a filter, called the glomerulus (pleural: glomeruli), and the tubules. Blood gets filtered in the nephron in two stages. First, the blood enters the glomerulus which is a cluster of miniature blood vessels with thin walls that serve as a sieve allowing large amounts of water, waste products and some nutrients to pass through while keeping larger molecules such as protein and blood cells in. In the second stage, filtered water, waste and nutrients enter tiny tubes (called tubules). These tubules help the body retrieve large portions of water and nutrients that were lost in the first stage while the waste gets excreted in the urine. This process is crucial for the body’s ability to regulate water, salt, minerals and acidity.
Nephron Endowment: The functional kidney mass at birth
Each kidney has about one million nephrons and filters about 150 liters of blood every day. Babies born at term have their complete number of nephrons. Adults cannot generate any new nephrons. However, as the body grows, the nephrons and the kidneys grow by enlargement of the glomeruli, tubules, and supporting structures. The number of nephrons a person is born with is called nephron endowment.
Many variables can lower that number including poor maternal health, diet or smoking during pregnancy. The use of steroids or alcohol, iron, vitamin A or vitamin D deficiencies during pregnancy were also linked to smaller child kidneys with a smaller number of nephrons at birth. It has been shown that a decrease in the nephron endowment is associated with a declinein kidney function and high blood pressure in adults. So, in summary, the number of nephrons at birth is a risk factor for kidney disease and high blood pressure.
Birth Weight and the Functional Kidney Mass
In most studies, reduced nephron endowment was associated with reduced birth weight. People who were born with a weight of <2.5 kg (<5.5 lbs) were found to be at higher risk for ending up with kidney failure requiring dialysis. In addition, preterm babies were also noted to be at an increased risk for decreased kidney function in adulthood. What happens is that with low nephron numbers at birth, the remaining nephrons enlarge and work harder leading to their accelerated aging and loss. This is worsened with any “catch up” after-birth growth and even obesity. Simply put, low birth weight is associated with a low number of nephrons at birth which increases the risk for kidney disease and high blood pressure.
The Role of Genetics and Epigenetics
The development of the human kidney is influenced by genetic and epigenetic factors. The deletion or alteration of a few genes has been associated with small kidneys and a decreased number of nephrons. The expression of some of these genes can be influenced by the nutritional health of the pregnant mother. What is more interesting is that the poor health of a pregnant mother not only affects the development of the kidneys of her children but also affects her grandchildren and further generations even if the new generations were born to well-nourished mothers. Throughout the world, small maternal stature is the most common risk factor associated with low birth weights. This is presumed to be caused by generations of nutritional deprivation and its effect on gene expression.[bctt tweet="The poor health of a pregnant mother not only affects the development of the kidneys of her children but also affects her grandchildren and further generations even if the new generations were born to well-nourished mothers" username=""]
Bottom Line
If you are born with low birth weight or preterm you are at a higher risk for kidney disease and high blood pressure. Nutritional, genetic and epigenetic factors play an important role. Modifying gene expression with nutrients in these circumstances might be helpful and is being researched.
References:
Hinchiffe SA, Sargent PH, Howard CV, Chan YF, Van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the dissector method and Cavalieri principle. Lab Invest. 1991; 64: 777-784
Ekblom P. Renal development. In The Kidney: Physiology and Pathophysiology (eds. Seldin DW, Giebisch G), 1992; pp. 475-501. Raven: New York.
Brenner BM, Chertow GM. Congenital oligonephropathy and etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994; 23:171-175.
Moritz KM, Cullen-McEwen LA. Kidney development and fetal programming. In Early Life Origins of Health and Disease (eds. Wintour EM and Owens JA), Advances in Experimental Medicine and Biology, vol. 573, 2006; pp. 130-144. Springer Landes Bioscience: USA.
Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004; 65:1339-1348.
Hoppe CC, Evans RG, Mortiz KM, et al. Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2007; 292: R462-469.
Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008; 19:151-157.
Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008; 19:151-157.
Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008; 19:2027-2034.
Quinlan J, Lemire M, Hudson T, et al. A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol. 2007; 18:1915-1921.
The Case Against Animal Protein for Kidney Disease
In December 2012, the international guidelines for the management of chronic kidney disease (also known as KDIGO) stated that restricting protein intake in patients with kidney disease remains controversial. Here we will discuss the case against animal protein for kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Case against animal protein for kidney disease
Interestingly, a low-protein diet was suggested as an approach for the treatment of kidney disease as early as 1869. It was thought that lowering protein intake leads to lower nitrogen waste products and lessens the burden on the kidneys. However, there have always been concerns about causing malnutrition and muscle wasting with lowering protein intake in kidney patients.
This was complicated after a large study in the nineties demonstrated that there is no benefit from a low-protein diet. Yet, that study was limited by its short follow-up on a disease that progresses over years and by excluding insulin-dependent diabetics and the use of high-tryptophan-containing keto acid supplements in patients with low protein intake. Nevertheless, it managed to cast doubt in the mind of many kidney doctors.
In this blog, I am going to explain why an excessive amount of animal protein is injurious to the kidneys and how to mitigate that.
Animal protein and kidney blood flow
An earlier hypothesis was that high protein intake might increase the burden on the diseased kidneys and leads to increased blood flow to the kidneys and pressure on the filtering units. This may lead to an increased generation of inflammatory molecules that fastens the progression of kidney disease. On the opposite, restricting protein intake in kidney patients improved blood pressure control, blood sugar control, and cholesterol control.
Animal protein and dietary acid load
In addition, there has been recent attention to blood acidity and its effect on the kidneys. Blood acid is a natural product of the metabolism of our food. Some foods produce a lot of acids and some produce less acid. The amount of blood acid produced by certain food is referred to as “dietary acid load.” A higher dietary acid load has been found to induce several injurious processes in the kidneys and leads to faster progression of kidney disease.
Indeed, supplementation with an alkali (Such as sodium bicarbonate, potassium citrate, or baking soda) has been found to slow down the progression of kidney disease. Natural alkali producers are fruit and vegetables, and studies have shown that adding 4-5 additional servings of fruit and vegetables to the standard American diet can decrease the acid burden on the kidneys.
On the other side, animal proteins produce some of the highest dietary acid load. The average American diet delivers approximately 15-17% of its energy as protein (mostly animal proteins) and is low in potassium-rich fruit and vegetables. For more information, read our blog about dietary acid load and CKD.
Animal protein and the gut microbiome
Furthermore, excessive animal protein intake may also lead to the predominance of bad bacteria in the gut. One of the most important regulators of gut microbiota (the balance of bacteria in the gut) is the type and availability of various nutrients in the diet, especially the ratio between undigested carbohydrates and proteins.
Healthy bacteria in the gut are important to maintain the intestinal barrier and prevent the seepage of endotoxins into the bloodstream. If the diet is rich in undigested carbohydrates such as fibers, proteins are mainly used for the growth of good bacteria that ferment carbohydrates. These bacteria produce short-chain fatty acids that provide food to the gut cells.
But when the diet has more protein than fibers, bad bacteria that ferment protein grow, leading to potentially toxic byproducts that get absorbed into the bloodstream and can lead to inflammation and further kidney injury. These byproducts are called uremic retention molecules (URM). For more information, read our blog on URM.
Animal protein and phosphorus intake
Animal proteins are also often associated with phosphorus in the diet. Phosphorus has many deleterious effects on the body and may lead to faster progression of CKD and worsening bone damage. Phosphorus can lead to inflammation in the blood vessels. It binds with calcium and deposits in various body tissues, including the skin, heart, and blood vessels. Excessive phosphorus can lead to the over-production of an inflammatory hormone that has been associated with heart disease and kidney disease.
There is no doubt that the literature strongly suggests that higher protein intake, particularly from animal sources, is associated with faster progression of kidney injury, and patients should consider decreasing their protein intake to 0.6 g/kg/day. Restricting protein intake below this level should be supplemented by essential amino acids or ketoacids.
References:
The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994, 330:877-84.
Dietary Protein Suppresses Feedback Control of Glomerular Filtration in Rats. J. Clin Invest. 1985, 75: 558-568.
Dietary Acid Load and Chronic Kidney Disease
In this blog we will discuss the role of dietary acid load on the progression of chronic kidney disease. But before we start let us talk about about blood acidity and how it is linked to our diet and how the body maintain it.
Blood acidity is strictly regulated in our body by intricate mechanisms that help maintain a range that is compatible with life. It is measured by what is called pH. The pH is technically a measure of hydrogen ion concentration. This term was utilized because hydrogen concentration is very miniscule and has been difficult to measure.
Maintaining blood acidity or pH level in a certain range is important for many minerals in our body to act properly and for the optimal function of many enzymes and hormones. Consuming the standard American diet (SAD diet) leads to the production of acid in the bloodstream due to metabolism of protein and other food elements, as seen in the figure below.
It is estimated that on average each person produces 1-1.5 mmoles of blood acid (hydrogen ion) for every kilogram of body weight per day. The acid produced by the metabolism is “buffered” by circulating alkali in the blood that is mainly produced by the kidneys. It is also buffered by our bones and by our muscles to some degree. The kidneys and lungs play a crucial role in maintaining blood acidity in the normal range.
Consuming a diet high in animal proteins that contains organic sulfur, cheese, eggs, and grains leads to the production of excessive amounts of blood acid that put an extra burden on the kidneys to excrete it. While consuming a diet high in fruits and vegetables produces blood alkali and has an opposite effect.
There are many ways to estimate the amount of acid produced by the diet. A good dietitian can easily make an estimate, but there are urinary tests that can help with getting a relatively accurate estimation.
In a study published in 2001, it was found that adding 4-5 additional servings of fruits and vegetables a day to the SAD diet can decrease acid load on the kidneys, without excessively restricting dietary protein.
Increased blood acidity has been found in the earliest stage of kidney disease. In fact, decreased handling of acid load was detected in as early as stage II kidney disease.
Increasing blood acidity has also been associated with a faster progression of kidney disease and correcting that with bicarbonate (an alkali) has been found to slow down the progression without significant side effects. In addition, accumulation of blood acid can lead to muscle wasting as the muscle proteins are used as buffer and also leads to bone resorption and osteoporosis. Supplementing elderlies with bicarbonate leads to increased strength and bone health.
Therefore, alkali therapy by sodium bicarbonate, potassium citrate, or simply taking baking soda has a positive protective effect on the kidneys and on the body in general. The same effect can also be achieved by limiting animal protein and processed food intake and increasing the intake of fruit and vegetables.
We recommend that a patient with kidney disease (and probably the general population) should limit the intake of animal protein, and add an additional 4-5 servings a day of fruit and vegetables (those on potassium restrictions should avoid these fruits and vegetables). We also recommend taking a quarter tea spoon of baking soda with a drink of choice twice a day. Each teaspoon of baking soda has 60 mmoles of bicarbonate which is much higher than any pharmaceutical tablet available at this time. Don’t forget to consult your Functional or Integrative medicine provider or nephrologist.
The Chronic Kidney Disease Epidemic
Kidney disease impact millions of Americans and is often associated with other diseases such as diabetes and elevated blood pressure (called hypertension). The statistics about the prevalence and outcomes of kidney disease come from the United States Renal Data System (USRDS) which publish a report every year. The most recent available report to date is the 2017 report. These data support that kidney disease reached epidemic proportions.
Overall, almost 15 percent of the US general population suffers from chronic kidney disease (CKD) with more than 35 million people affected in the US and greater than three million in Canada. In other words, 1 in seven people reading this blog have some form of CKD. Diabetes and hypertension are the two main causes of CKD. Together they are responsible for more than %70 of t CKD. Unfortunately, as of December 31, 2015 more than 700,000 Americans developed kidney failure (also called End Stage Renal Disease or ESRD) requiring dialysis or kidney transplant. Women are slightly more likely to have stages 1 to 4 CKD. In addition, race and socioeconomic status plays an important role.
Stage 3 is the most common kidney disease
Sadly, kidney disease is called a “silent disease” because it has no symptoms early on and can go undetected until it is very advanced. On top of that, many of these patients have a hidden heart disease along with their kidney damage. Each year, kidney disease kills more people than breast cancer or prostate cancer. In 2015, more than 95,000 Americans died from kidney disease. Despite these staggering numbers, you do not see any effective national campaigns to raise awareness of kidney disease. Yet there is a glimmer of hope as the rate of new cases of ESRD has declined slightly since 2006.
But despite that, the prevalence of ESRD continues to rise because dialysis and kidney doctors are doing better job at keeping these patients alive and because the prevalence of type 2 diabetes continues to be on the rise.
Kidney disease is also costly. It is estimated that Medicare spending for patients with CKD ages 65 and older exceeded $55 billion. This is 20 percent of all Medicare spending in this age group. While dialysis patients in the US constitute 1% of the Medicare populations they account for 7% of its expenditure with up to $31 billion a year.
Conventional medicine has focused on the management of kidney disease, diabetes, blood pressure control and lipid control but so far this did not lead to the reversal of the CKD epidemic.
There is an urgent need to raise awareness of this silent killer and pursue an integrative medicine approach that pay attention to genetic predisposition, environmental exposure, diet and lifestyle modifications.
In the next blogs, we will talk about inflammation and the role of the gut and the “gut-kidney axis” in kidney health.