Stress and kidney health
Chronic kidney disease (CKD) is rapidly becoming a global health problem. The prevalence of CKD remains high and the incidence of end stage kidney disease or kidney failure continues to rise. According to the Centers for Disease Control and Prevention (CDC), more than 1 in 7 US adults are estimated to have kidney disease. Many factors contribute to the increase in CKD prevalence, including psychological stress (we will refer to this as “stress”). In this blog, we will discuss the link between stress and kidney health.
Types of Stress
Generally speaking, there are three types of stress:
- Major catastrophic life events that require considerable behavioral modification such as the death of a loved one, divorce or loss of a job.
- Chronic strains that are persistent stressors which require some adjustment over a long period of time such as poverty or disability.
- Daily hassles that occur over the course of the day such as traffic issues or unpleasant personal interactions.
The way people perceive and handle stress varies from person to person, with some people better equipped to handle stress than others. Certain factors, which we detail below, impact a person’s ability to cope with stress. These include genetics, environment, and in utero exposure. Furthermore, previous traumatic life experiences may affect future handling of stress in an individual.
Most of the early literature on stress focused on the first two types listed above, but research is now looking at the third type and its effect on health.
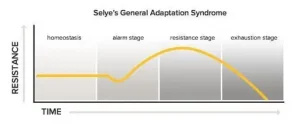
According to Selye, there are three phases of behavioral adjustments to stressors. The first phase is the alarm stage in which a person will have a “fight or flight” response. If the stressor persists, the person enters into the resistance or adaptation stage. Finally, long term exposure to the stressor will eventually lead to the exhaustion stage.
Factors that influence stress
Although we all experience stress, some individuals are better suited to handle stress than others. There may be some individual variability due to various genetic and environmental factors, as well as the impact of trauma and adverse childhood experiences.
Genetics
Small differences in our genes, called single nucleotide polymorphisms (SNPs), can affect our hormonal responses and enzymes that are associated with psychological responses. One example is a SNP in catechol-O-methyltransferase (COMT) gene which produces an enzyme that breaks down dopamine, epinephrine, and norepinephrine (neurotransmitters associated with the fight-or-flight response). Mutations in the way this gene is expressed, can lead to an increased stress response.
Another example is a protein called brain-derived neurotrophic factor (BDNF) which protects and improves signaling between brain cells. Any SNPs in BDNF or its receptors can decrease resilience to stress. In fact, the impact of stress on BDNF has been strongly associated with affective disorders.
Environment
The area and physical environment where a person lives and works can be a stressor. Environmental stressors such as living in disadvantaged neighborhoods, limited access to supermarkets, residential density, availability of recreational resources, and distance to wealthy areas, are associated with the development of insulin resistance, type 2 diabetes, obesity, and cardiovascular disease, all of which are risk factors for CKD. It is important to know this is subjective and environmental stressors for one individual may not be stressful to another.
In Utero Exposure
The stress a woman experiences during pregnancy can greatly impact the development of the baby while in utero, leading to lifelong complications. Prenatal stress has been shown to affect hypothalamic-pituitary-adrenal (HPA) axis programming of the fetus, making it more difficult for the offspring to handle stress in the future. Furthermore, this altered HPA activity has been linked to low birth weight which may result in a decrease in nephron endowment (the number of filtering units the baby is born with), as well as metabolic syndrome and cardiovascular disease, known risk factors for CKD.
Nutrition as a Stress Mediator
Research suggests that some nutrients can heighten the response to stressors while other nutrients relieve it. For example, magnesium and zinc can help a person feel more calm. In addition, omega-3 fatty acids and foods high in probiotics can reduce stress and anxiety. On the other hand, caffeine can make a person feel anxious. It is important to note that genetics play a role in caffeine metabolism and its effects.
Join us to end the kidney disease epidemic
Stress and Kidney Health
Indirect Effect of Stress on the Kidney
Stressors can lead to changes in the hypothalamic-pituitary-adrenal axis (HPA axis) and neuroendocrine hormones. These changes are associated with increased cortisol levels, increased epinephrine, and inflammatory cytokines which are linked to the development of insulin resistance, metabolic syndrome, high blood pressure and type 2 diabetes.
Direct Effect of Stress on the Kidney
Studies have shown that the sympathetic system innervates all segments of the kidneys including the blood vessels and tubules. Stimulating the sympathetic system can affect blood flow to the kidneys and lead to sodium retention at the level of the tubules, resulting in the progression of kidney disease.
The Vicious Cycle
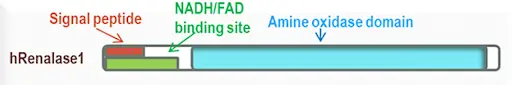
As we have seen above, epinephrine and norepinephrine can play a role in the psychological stress response on the kidneys. These neurotransmitters are usually broken down in our bodies by two major enzymes: monoamine oxidase (MAO) and COMT. Recently, a new enzyme, renalase, was found to be produced by the kidneys and involved in the breakdown of these neurotransmitters.
Renalase is a flavoprotein that metabolizes catecholamines (epinephrine, norepinephrine, and others) to aminochromes. It utilizes NADH as a cofactor. Therefore, deficiencies in vitamin B2 and B3 can affect its function. Studies have shown that renalase levels in the blood correlate with normal kidney function and kidney mass. Decreased level of renalase leads to elevated blood pressure and heart rate, physiological responses to stress. Furthermore, it’s been shown that decreased levels of renalase increase the sensitivity of the heart and the kidneys to ischemia (decreased blood flow).
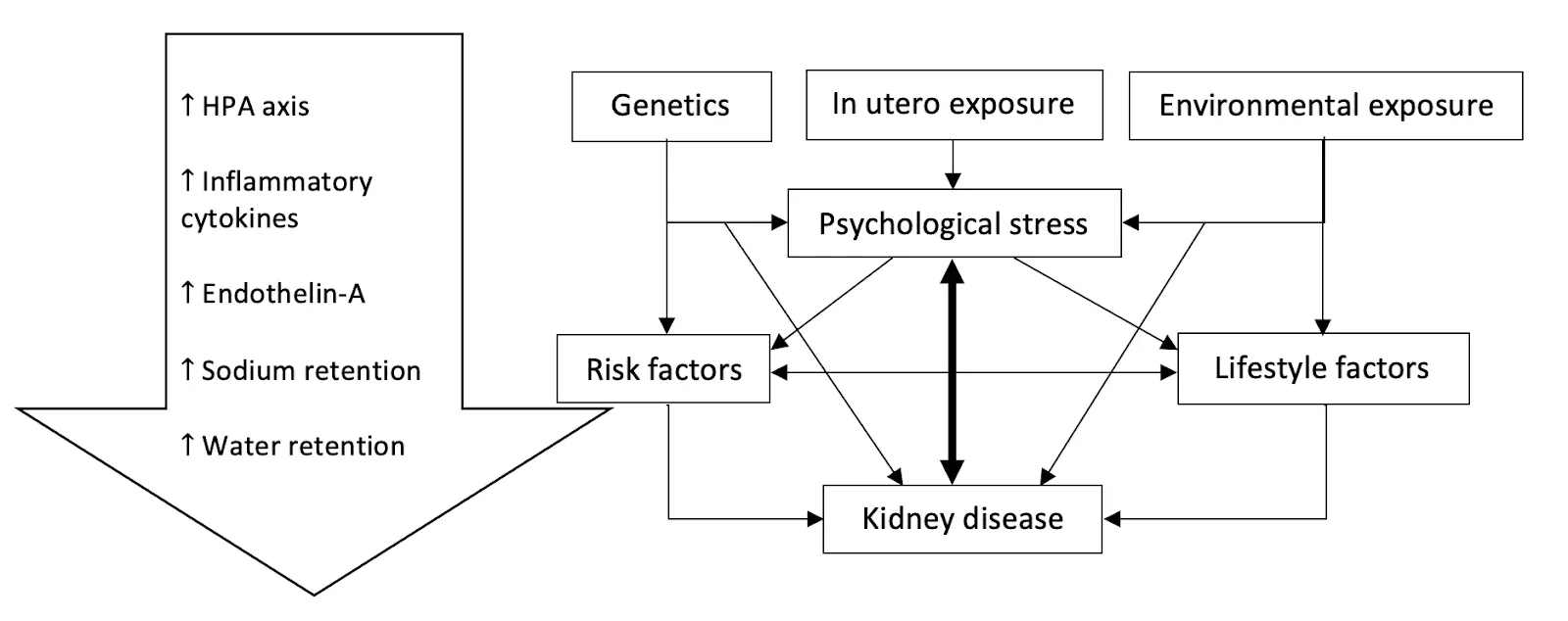
This creates a vicious cycle in which stress causes kidney disease, and the latter, in turn, amplifies the effect of stress on the body.
In essence, the interplay between stress and kidney disease can be summarized in this diagram.
The Bottom Line on stress and kidney health
Our increasingly stressful lives can lead to increased cortisol levels, insulin resistance, and inflammation. Stress has been linked to increased levels of epinephrine and norepinephrine. These changes lead to the development and progression of kidney disease. Genetics, environment, and nutrition all play important roles in augmenting these changes. It’s essential that a stress management practice is part of a comprehensive Integrative and Functional Medicine approach to kidney health.
Clinical Utility of Genetic Testing for Kidney Transplant Evaluation
Genetic testing for patients with kidney disease can have a remarkable impact on their care. The availability of “broad-panel genetic testing” for kidney patients ushers in a new era of nephrology and patient care. Tests that used to cost thousands of dollars and took months for results can now be done for a fraction of the cost in just a few weeks. New commercially available genetic tests utilize next-generation sequencing to identify multiple gene variants simultaneously. These tests can help in the management of kidney disease in multiple ways. In this blog, we will focus on the clinical utility of genetic testing for kidney transplant evaluation.
Genetic Testing for Kidney Transplant Evaluation
During the evaluation of a patient for a kidney transplant (the recipient), the assessment usually focuses on answering these questions:
- Can the recipient survive elective transplant surgery?
- Can the recipient tolerate immunosuppression after the transplant?
- Can the recipient have a good outcome?
In addition, evaluation of living donors try to answer questions about their suitability for donation and their risk of developing kidney failure in the future.
Living Donor Evaluation
One of the most pressing questions when evaluating a living donor is: Will this donor develop kidney disease in the future if s/he donates a kidney now? Several studies have shown an increased risk of the donor developing kidney disease after donation. This risk is higher if the donor and the recipient are related. This may indicate that genetic factors play a role in this risk.
In 2017, the Kidney Disease Improving Global Outcomes (KDIGO) Guidelines suggested that “transplant programs should have a strategy for evaluating for inherited kidney disease in donor candidates when there is a family history of kidney failure and the recipient’s cause of kidney failure is uncertain.”
These guidelines suggested genetic testing of living related donors with specific diseases such as focal segmental glomerulosclerosis (FSGS), atypical hemolytic uremic syndrome, Alport disease, sickle cell trait, and autosomal dominant tubulointerstitial kidney disease.
Genetic testing of a living relative donor can be especially important if the recipient has polycystic kidney disease. If this mutation is identified in the recipient, the donor can then be tested and excluded if s/he has the mutation. This can give greater assurance to both the donor and recipient.
Other genetic variants are associated with increased risk of chronic kidney disease (CKD) such as APOL1 gene variants that are associated with increased risk for nephropathy in patients of African ancestry. Incorporating testing for these genetic risk variants in the evaluation of the donor may help replace race for calculation of the so-called Kidney Donor Risk Index that is used to predict the longevity of the transplant graft.
While it is still too early to incorporate the genetic risk variants for diabetic kidney disease and IgA nephropathy in transplant evaluation, getting more clarity on the utility of the risk variants can have a tremendous impact on the care of current patients.
Recipient Evaluation
Kidney disease is silent in its progression and symptoms do not develop until the advanced stages of CKD. One in 10 patients with advanced kidney diseases presents with end-stage kidney disease (ESKD). In many of these cases, the laboratory workup is inconclusive, and their kidneys are often too atrophic to biopsy. Unlike kidney biopsies, genetic data can be informative even after ESKD has developed.
Genetic evaluation of the recipient is, therefore, helpful in identifying the causative mutation that could have led to the disease. Using targeted gene testing, researchers were able to identify pathogenic mutations in 19% of waitlisted transplant patients under the age of 40. Broad panel genetic testing can likely have an even higher yield. Indeed, broad panel genetic testing has been shown to identify the cause of CKD in up to one-third of the patients with an unknown cause.
Genetic testing of the recipient can also help in providing individualized post-transplant care. Finding a specific mutation that leads to a localized disease in the kidneys can decrease concerns about the recurrence of the disease after transplantation.
Also, a genetic diagnosis can often point to the likelihood of disease in another organ and can prompt referral and evaluation.
Currently, researchers are collecting phenotypic and genetic information on patients receiving transplants in the iGeneTRAiN consortium. Analyzing this data in the future may have a significant impact on our understanding of transplant graft outcome.
Pharmacogenomics
Wouldn't it be a relief to be able to predict in advance how someone might respond to a medication? This would save time, eliminate guesswork, and improve patient outcomes. Thanks to advances in a field of genetics called pharmacogenomics (PGx), clinicians have begun to use genetic information to personalize drug therapy.
Accurate pharmacogenomics data are now available on two transplant medications: tacrolimus and azathioprine. Although the latter is not commonly used, the former is used often. Tacrolimus is metabolized by the enzyme encoded in the gene CYP3A5. Variants in this gene can classify the patient into one of three phenotypes: extensive metabolizer, intermediate metabolizer, and poor metabolizer. Indeed, pharmacogenomic data can now be used to optimize the initial dose of tacrolimus.
Many other medications commonly used by patients have pharmacogenomic data which can also be used to optimize their dosing. Medications such as clopidogrel, voriconazole, and allopurinol are a few of these. We discussed these medications in-depth in our previous blog about pharmacogenomics.
The Bottom Line
Genetic testing is gradually becoming a significant part of the transplant evaluation of the donor and the recipient. It is particularly useful in the evaluation of living donors with a family history of kidney disease. This data has the potential to transform the care of kidney transplant patients and improve their outcomes.
May Research and News
Sex-specific Associations of Sex Hormone Binding Globulin with CKD and Kidney Function: A Univariable and Multivariable Mendelian Randomization Study in the UK Biobank
Do sex hormones play any role in the progression of kidney disease? It has been long observed that CKD is more prevalent in women; however, the progression of CKD is much faster in men. This study looked at the sex hormone binding globulin (SHBG) using Mendelian randomization. Higher SHBG levels were associated with lower risk for CKD and better renal function in men but not in women. "Identifying factors affecting SHBG, and underlying pathways, could provide new insights for prevention and treatment strategies."
https://jasn.asnjournals.org/content/32/3/686
Water intake and progression of chronic kidney disease: the CKD-REIN cohort study
How does water intake affect the progression of kidney disease? In this study, researchers found that "in patients with CKD, the relation between plain water intake and progression to kidney failure appears to be U-shaped. Both low and high intake may not be beneficial in CKD."
https://academic.oup.com/ndt/advance-article-abstract/doi/10.1093/ndt/gfab036/6134139?redirectedFrom=fulltext
Anti-neutrophil cytoplasmic antibody associated glomerulonephritis complicating treatment with hydralazine
Medications can sometimes be triggers to autoimmune kidney diseases. This article talks specifically about hydralazine which is an old blood pressure medication that is used often by our cardiology colleagues specifically in patients with advanced kidney disease who are often not candidates for ACE inhibitors or ARB therapy.
https://www.kidney-international.org/article/S0085-2538(21)00379-3/fulltext?WT_MC_ID=ITL
Dietary Phosphorus and Kidney Health
One of the most harmful nutrients to the kidney is phosphate. Yet, the body needs phosphate for many essential biological functions. In this blog, I will discuss and clarify the role of dietary phosphate in kidney health. I will use the term phosphorus instead of phosphate for ease.
Daily requirements of phosphorus
There is no doubt that the average person requires phosphorus in the diet to live a healthy life. On average, the normal person requires 900-1250 milligram of phosphorus a day. Higher intake is especially required for growing children and pregnant women. Unfortunately, the standard American diet often contains higher amounts of phosphorus.
Role of phosphorus
Phosphorus plays an essential role in bone and teeth formation. It is also required for metabolism and energy production in the body. It is crucial for normal heart and nerve functions. Phosphorus serves as a building block for many structural and functional proteins. Because of its important role, the gut, hormones, kidneys, bones, and even vitamin D are involved in regulating phosphorus levels in the body.
The different types of dietary phosphorus
To simplify, we divide dietary phosphorus into two major categories. The first is organic phosphorus which comes "naturally" from animal and vegetarian proteins. The second is inorganic phosphorus, which is present in food additives.
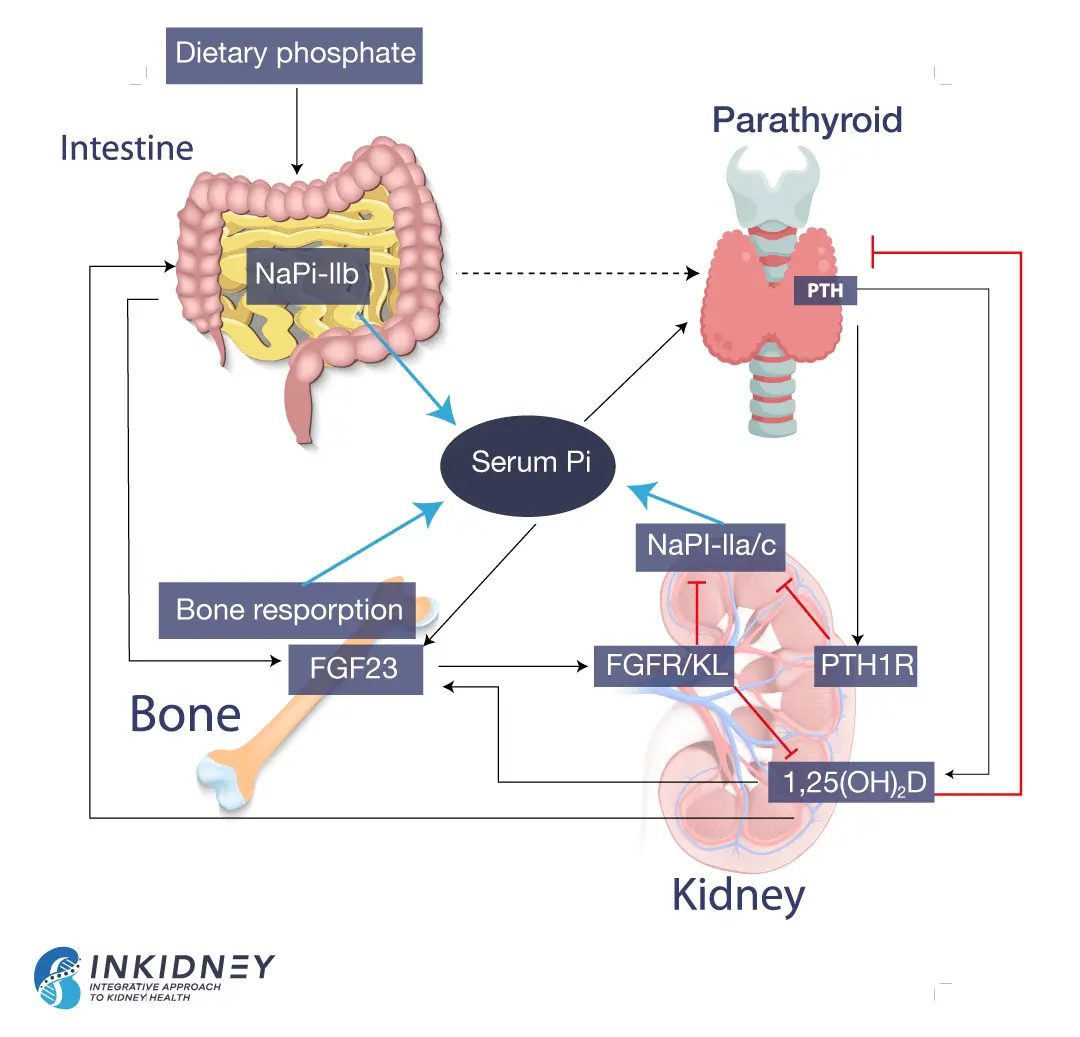
How the kidneys handle phosphorus
The kidneys are critical for keeping a balanced, steady state of phosphorus in the body. Most of the phosphorus ingested in food gets absorbed in the gut. Some of it leaves the body in the stool. Circulating phosphorus gets filtered by the glomeruli. In this process, tiny capillaries in the kidney take phosphorus out of the blood. However, the kidney tubules help reclaim that phosphorus. They add phosphorus back to the blood, a process called reabsorption. The glomeruli and kidney tubules work together to filter waste out of the blood, but also recycle nutrients that are needed, like phosphorus. The end result is that the amount of phosphorus excreted in the urine equals that absorbed in the gut.
Hormonal control of phosphorus balance
The reabsorption of phosphorus in the kidneys depends on several hormones. The parathyroid hormone (PTH) decreases phosphorus reabsorption. When phosphorus isn’t recycled, more phosphorus leaves the body through the urine. On the other hand, 1,23 (OH) vitamin D increases phosphorus reabsorption, meaning that the body holds on to more phosphorus. Vitamin D also increases phosphorus absorption by the gut.
Bones also influence phosphorus levels. They produce fibroblast growth factor 23 (FGF-23), which decreases phosphorus reabsorption. Simply put, FGF-23 tells the kidneys to excrete phosphorus in the urine, instead of holding on to it. The following picture summarizes phosphorus balance.
Phosphorus in kidney disease
In kidney disease, the ability of the kidney to excrete phosphorus progressively declines. Data shows that the decline begins early in kidney disease. In response, there is an increase in the production of PTH and FGF-23. Both hormones attempt to rid the body of phosphorus by decreasing the reabsorption of phosphorus by the kidneys. In the beginning, this effort maintains normal phosphorus in the blood. But as kidney disease advances, the ability of the kidneys to excrete phosphorus decreases further. Thus, in advanced kidney disease both PTH and FGF-23 are high, but phosphorus accumulates in the blood.
Elevated phosphorus binds to calcium and deposits in the blood vessels and under the skin. This leads to inflammation. In addition, several studies have shown that elevated levels of PTH and FGF-23 are associated with poor outcomes in kidney patients. Elevated levels of FGF-23 lead to poor cardiovascular outcomes and progression of CKD. Elevated PTH causes poor bone health in kidney patients.
Dietary phosphorus in kidney disease
Recent data showed that patients on plant-based diet are less likely to develop kidney disease. In fact, a plant-based diet is now recommended for kidney disease patients. Some worry that plant-based diets lead to higher intake of phosphorus. Many practitioners believe that a diet high in legumes, nuts and seeds will increase intake of phosphorus.
Yet, when we look at serving size, animal protein and dairy have the highest content of phosphorus. Besides, phosphorus in vegetarian sources is bound to phytic acid. Humans don't have the enzymes needed to digest phytic acid. So, we actually do not absorb a lot of the phosphorus that is present in plant-based foods.
Actually, we only absorb 40% of vegetarian phosphorus as compared to 60% of animal-based sources of phosphorus.
But the food industry is actually adding phosphorus to many foods. This is part of food processing. Some use it as a preservative. Some use it to prevent discoloration. Others use it to improve taste. You often see names that are not easily recognizable. These names include calcium phosphate, disodium phosphate, phosphoric acid, or sodium triphosphate. These types of phosphorus are the inorganic phosphorus. We absorb almost 100% of phosphorus as food additives.
This is why it is so important to look at the ingredients. Any ingredient that has the four letters, “Phos” in it means that it contains phosphorus. You can find great resources about dietary phosphorus here.
The bottom line
Higher intake of dietary phosphorus leads to worse kidney outcomes. Processed food has the highest absorbable phosphorus, followed by animal proteins. Vegetarian sources have the lowest absorbable phosphorus. This supports the use of a whole foods, plant-based diet in kidney disease.
FAQs About Genetic Testing for Kidney Disease
Genetic testing for patients with kidney disease can have a tremendous impact on their care. The term “broad-panel genetic testing” is used to describe commercially available genetic tests that utilize next generation sequencing. These tests offer numerous advantages in the management of kidney disease. However, because genetic testing is fairly new, many patients and providers are hesitant to order these tests. In this blog, we will answer some of the common FAQs about genetic testing in adults with kidney disease.
FAQs about genetic testing for kidney disease
What can I learn from genetic testing?
Genetic testing for kidney disease can help diagnose unknown causes of chronic kidney disease (CKD) and help identify changes in the genome that may increase CKD risk. Results from the tests can even help providers correct improper diagnoses. In addition, genetic testing can determine kidney disease severity. Identifying specific gene variants can help guide treatment for specific types of CKD as well as proper dosing of medications.
How does it work?
In order to perform the test, a sample of DNA is required, usually obtained through a saliva or blood sample. The cells from the sample are processed and analyzed in a database, obtained from Genome Wide Association studies (GWAS), of currently known genetic variants associated with CKD. These studies looked at multiple genetic variants and linked them statistically to specific kidney diseases in established patients.
Does genetic testing help diagnose specific kidney disease?
As mentioned above, genetic testing can help identify genetic variants associated with certain kidney diseases. However, carrying a genetic variant does not necessarily mean a person will develop the disease. Other factors can play a role in the genes’ ability to express themselves. Genetic tests should always be interpreted in consultation with an experienced nephrologist or genetic counselor.
Will these tests identify all genetic variants associated with CKD?
No, our knowledge about the genetic basis for CKD is still evolving. To date, mutations in more than 500 genes have been associated with different forms of kidney disease. The specific genetic mutations identified on the panel varies depending on the genetic testing company used. In addition, genetic testing cannot tell you everything about inherited diseases. Diet, lifestyle, and environment all influence how genes are expressed, a field known as epigenetics.
Why do I need genetic testing for kidney disease if I am already doing regular physicals and lab checks?
Kidney disease is a silent disease and patients usually don’t develop symptoms until later stages of CKD. In addition to laboratory testing, genetic testing provides information for those at increased risk of CKD. Knowing your genetic risk provides you with an opportunity to modify your lifestyle in order to decrease the chance of kidney disease and failure in the future.
Is this test covered by my health insurance?
Depending on the test performed, your insurance may or may not cover a portion of the cost. Some small panel tests implemented by a few pharmaceutical companies are free. These companies hope to recoup the cost by identifying patients who will benefit from their products. In general, broad panel genetic tests are now much more affordable than they used to be.
Will I be discriminated against based on my results?
Federal law prohibits health insurance providers and employers from discriminating against a person based on genetic information. However, unfortunately, this law does not apply to long-term care, disability, or life insurance providers. It is crucial that you choose a testing company that values your privacy.
The Bottom Line
Genetics are one factor that plays a significant role in the development of kidney disease. Genome-wide association studies (GWAS) have identified several hundred genes that are associated with kidney diseases. Therefore, genetic testing may play a crucial role in the management of kidney disease patients.
April Research and News
Association of Physical Activity and Poor Health Outcomes in Patients With Advanced CK
Here, it was found that physical activity can improve outcomes for kidney patients. Higher level of physical activity was associated with 52% reduction in all cause mortality in advanced CKD patients. This is better than any medication that we know.
See the full article here.
Effects of antihypertensive medication and high-intensity interval training in hypertensive metabolic syndrome individuals
In this study, researchers looked at the effect of the combination of antihypertensive medication and exercise training on blood pressure control in patients with metabolic syndrome. Exercise training proved to have additional blood pressure lowering to medications targeting the renin angiotensin aldosterone system (RAAS). However, exercise did not affect RAAS hormones level or urine protein level.
https://pubmed.ncbi.nlm.nih.gov/33662166/
Proton pump inhibitors associated acute kidney injury and chronic kidney disease: data mining of US FDA adverse event reporting system
The evidence is piling up about the association between the use of proton pump inhibitors (PPIs) and kidney injury. Here, investigators studied FDA adverse reporting data and found further evidence to link PPI to kidney injury. The strongest association was with Dexlansoprazole.
https://pubmed.ncbi.nlm.nih.gov/33574396/
Identification of a novel interplay between intestinal bacteria and metabolites in Chinese patients with IgA nephropathy via integrated microbiome and metabolome approaches
Is there any link between dysbiosis and IgA nephropathy? This study from China shows that gut of patients with IgAN have higher levels of Streptococcus and Enterococcus and lower levels of Bacteroidetes and Bacteroides. The study also showed mulitple metabolites that generate from the gut microbiota and are associated with IgAN
https://pubmed.ncbi.nlm.nih.gov/33553325/
Genetic testing in kidney disease
Current evidence suggests that genetics play a role in the development of kidney disease. Common genetic disorders associated with kidney disease include polycystic kidney disease, Alport’s Syndrome and Fabry’s disease. The advances of genome-wide association studies (GWAS) helped identify several hundred other genes linked to kidney diseases. This made genetic testing a useful tool in the management of kidney disease patients. In this blog, we will detail the benefits of broad-panel genetic testing in kidney disease management.
Next Generation Sequencing
Various methods for identifying genetic variants have been used in the past. The exome is that 2% of the genome that codes for all biological proteins. Next generation sequencing is a new technology that allows DNA sequencing of the entire human exome within a single day. It also captures a broader spectrum of variations that can affect the genetic code. This revolutionary technology can identify mutations associated with CKD. It can also recognize variants associated with an increase in risk or severity of CKD. It is now available for kidney disease patients. This type of “broad-panel genetic testing” can transform care for patients with kidney disease.
Clinical benefits of genetic testing in kidney disease management
Identify diagnosis of unknown causes
While diabetic and hypertensive kidney diseases are the most common causes of kidney failure, there are others. There are instances when providers find themselves unable to identify the cause of kidney disease. Indeed, in some cases, urinalysis is actually “bland” and the workup to identify the cause of kidney disease is negative.
Even a kidney biopsy may not be helpful. It may show glomerulosclerosis and fibrosis (scarring). This does not help identify the original cause of kidney disease. Genetic testing can have a tremendous value in these cases. In fact, whole exome sequencing was able to diagnose up to one third of the patients who had unknown causes of kidney disease.
Reclassify a clinical diagnosis
It is common for providers to label kidney disease patients with unknown causes as hypertensive kidney disease or “nephrosclerosis”. This is because hypertension is common in kidney disease patients. Studies have shown that 60-90% of patients with chronic kidney diseases have high blood pressure. It is often not clear which came first, hypertension or kidney disease. It is possible that patients who were diagnosed with hypertensive kidney disease have another cause. In fact, studies have shown that up to a quarter of kidney diseases can be reclassified with a broad-panel genetic testing.
Target the therapy and workup
Broad-panel genetic testing can help providers avoid unnecessary procedures, tests, and treatments. It gives accurate and “molecular level diagnosis”. It provides a better idea of the outcome of the specific kidney disease. It also helps providers avoid the use of immunosuppressive medications in patients with genetic causes of kidney disease. Furthermore, it can guide therapy for specific genetic causes that we currently have treatment for such as Fabry’s disease. This type of testing may, indeed, eliminate the need for a kidney biopsy.
Role in kidney transplant
Genetic testing can have a tremendous impact in guiding kidney transplant and in the care of kidney transplant recipients. Kidney transplant donors can be pre-screened by broad panel genetic testing to assess if they carry any genetic kidney disease risk. While carrying the genes does not necessarily indicate that the donor will have the disease, it can play an important role in selection. This is especially true for living donor kidney transplant when the recipient has a known genetic kidney disease, and the donor is too young to have any manifestations.
In addition, many immunosuppressive medications that are used by kidney transplant patients are metabolized by well-established pathways that can be affected by genetic SNPs.
Having this genetic information can help providers prescribe the proper dose of immunosuppressive medications which is critical in transplant patients to avoid rejection or toxicity. This evolving field is called pharmacogenomics.
Help with management of kidney patients
Broad panel genetic testing can identify patients who are at risk for kidney disease, reclassify the exact cause of kidney disease, and guide treatment. Knowing the molecular basis of some kidney diseases can guide lifestyle modification interventions, future development of drugs, and gene therapy. It can also identify early complications outside the kidneys that can be related to the specific genetic disease and prompt early interventions.
Available broad panel genetic tests
Broad panel genetic tests are now available for providers and patients, and are relatively inexpensive. Many of them are covered by insurance companies. We have utilized Natera’s Renasight broad panel genetic test for this purpose. It provides next generation sequencing for 382 genes that are associated with kidney disease. There are also other genetic testing companies that test a small panel of genes for free to identify patients for drug or gene therapy.
The Bottom Line
We are at the dawn of a new era in nephrology and kidney care. Broad panel genetic testing will revolutionize kidney disease management. Our genes are not our destiny, but we cannot change our destiny without knowing our genes.
March Research and News
Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease
Does elevated uric acid blood levels without symptoms of gout affect the progression of kidney disease?
A new study tried to answer this question. Previous data suggested that elevated asymptomatic uric acid could be a risk factor for the progression of CKD. However, a recent study published in NEJM last year 6/2020) found no benefit from lowering uric acid using allopurinol to slow the progression of CKD.
This new study found that it is not about the level of uric acid in the blood but about the presence of uric acid (UA) crystals in the urine. UA crystallizes in the kidney. UA crystals contribute to CKD progression because UA crystals trigger M1-like macrophage-related interstitial inflammation and fibrosis.
This study points to the limitation of conventional research that doesn’t look into the individual characteristics of each patient and point to the importance of using the simple and inexpensive urinalysis to help guide risk assessment and approach to managing uric acid in CKD.
https://jasn.asnjournals.org/content/31/12/2773
Association Between Midlife Physical Activity and Incident Kidney Disease: The Atherosclerosis Risk in Communities (ARIC) Study
Fresh from January’s American Journal of Kidney Disease:
Physical activity decreases the incidence of kidney disease... not surprisingly
Making lifestyle changes can be challenging, but are an important part of the fight against kidney disease. It can be a challenging process but understanding the brain’s efficacy and patterns can help individuals make the changes needed to lead a healthier lifestyle. Read more on our blog.
https://pubmed.ncbi.nlm.nih.gov/32971191/
Effect of Kidney Function on Relationships between Lifestyle Behaviors and Mortality or Cardiovascular Outcomes: A Pooled Cohort Analysis
In this study, investigators found that lifestyle modifications can reduce the risk of cardiovascular disease in patients with kidney function. They looked at smoking cessation, maintaining normal BMI, physical activity, healthy diet and moderate consumption of alcohol individually and in combination. Beside smoking cessation and physical activity, other individual lifestyle modifications were not sufficient alone according to this study. This emphasizes the importance of an integrative approach to kidney health and disease.
https://jasn.asnjournals.org/content/early/2021/02/04/ASN.2020040394
Laxative Use and Risk of Dyskalemia in Patients with Advanced CKD Transitioning to Dialysis
In this article, researchers found that using laxatives alone can help maintain potassium balance in patients with advanced kidney disease. The colon can actually play an important role in potassium excretion. That is one of the many reasons why a diet high in fiber is essential for kidney patients.
https://jasn.asnjournals.org/content/early/2021/02/04/ASN.2020081120
Lead Exposure and Kidney Health
About 700,000 individuals are affected by chronic kidney disease (CKD) throughout the world. In fact, according to the CDC, 1 in every 7 people in the US have CKD. The majority of risk factors for CKD are related to lifestyle factors or conditions related to lifestyle disease. These lifestyle factors include impaired fasting glucose, elevated blood pressure, high body mass index, a high sodium diet and exposure to lead. In this blog, we will focus on the link between lead exposure and kidney health.
Although there has been a considerable reduction in lead exposure over the last few decades, low level exposure is still common, globally. Concerns about chronic low-level lead toxicity are associated with gradual accumulation of the heavy metal in the body.
Sources of lead exposure
Lead contamination can be due to exposure to various household products, water, soil, and air. Most significant sources of lead include:
- Homes built before 1978 (when lead-based paints were banned) probably contain lead-based paint.
- Water pipes may contain lead (recently we learned about the impact on which can lead to contamination of community water crisis as it has happened in Flint, MI).
- Some toys
- Jewelry
- Cosmetics
- People involved in jobs and hobbies working with stain glass, ceramic glazing, welding and batteries
- Aviation fuel (living near airports may increase risk of exposure in air and soil)
It is estimated that a large population of adults and children in the United States have blood lead levels that are higher than what is considered safe.
How does the body handle lead?
When it enters the GI tract, lead utilizes the iron gates (Divalent metal transporter (DMT1)) in the gut to enter systemic circulation. In the case of mineral deficiency, especially iron deficiency, lead can more easily compete for absorption via DMT1. This is significant, considering the low mineral status of the Standard American Diet (SAD). Furthermore, lead can compete with iron for incorporation into heme by inhibition of an enzyme called 6-aminolevulinic acid dehydratase (ALAD), increasing risk of iron-deficiency anemia.
Lead cannot be converted by the body, and is either stored in tissue (primarily in bone, replacing calcium) or eliminated (primarily through the kidneys and gut). It can accumulate and remain stored in the bones for decades, and is released into the blood under certain circumstances such as pregnancy, breast-feeding, menopause, weight loss and advancing age.
How the kidneys handle lead
Lead competes with iron and calcium due to similarities in atomic size. After lead is filtered by the glomeruli, it enters the proximal tubular cells via calcium channels. In fact, lead is 10 times more efficient at utilizing calcium channels than calcium itself. Some of it returns to recirculate in the blood while some is transported back to the tubular lumen through other transporters. This constant exposure causes damage to the tubules and increases oxidative stress by using up glutathione, an antioxidant.
The effect of lead on the kidneys
The kidneys are the major site of elimination for lead and appear to be one of the primary sites of accumulation. Even exposure to small levels of lead early in life can lead to glomerular hypertrophy and may disrupt glomerular development leading to renal insufficiency later in life.
However, the majority of lead toxicity in the kidneys is due to its effects on the tubules. Chronic exposure has been shown to lead to progressive tubulointerstitial nephritis including inflammation, fibrosis, and atrophy. The mechanism involves oxidative stress, mitochondrial injury, and acting as a functional substitute to calcium and increasing unchecked activity of protein kinase C. Lead can also replace calcium in the cellular tight junctions compromising its integrity. Finally, lead decreases the ability of the tubules to eliminate uric acid.
Collectively, these lead to the classic triad of lead exposure, which includes progressive kidney disease, elevated blood pressure, and gout.
Nutrients and lead exposure
It makes sense now to understand that a deficiency in iron and calcium can lead to an increase in lead toxicity. It has been documented that the absorption of lead in the gut is actually inversely proportional to dietary levels of calcium. Studies in children demonstrated a relationship between dietary calcium and blood levels of lead by linking a low dietary intake of calcium to higher lead levels. Indeed, increasing dietary calcium intake has been shown to decrease blood levels of lead.
Genetics and lead exposure
Genetic mutations in iron and calcium metabolism can greatly impact lead toxicity. In fact, genetic mutations in the gene coding for ALAD have been shown to affect blood and bone lead levels. In addition, polymorphisms in the gene for vitamin D receptor (VDR) were found to influence the accumulation of lead in bone. VDR is involved in calcium absorption from the gut and its placement in bone.
Finally, variations in the hemochromatosis gene that codes for the HFE protein associated with iron absorption can also increase the risk of lead absorption. These genetic variations can increase an individual’s susceptibility to lead toxicity even from relatively low-level exposure. In other words, what may be a “safe” exposure level to one person may be a toxic level to another depending on genetic variables, especially when combined with other lifestyle or occupational factors.
The microbiome and lead exposure
Metabolomics is the study of the substrates and byproducts of metabolism of various microbes found in the body and their impact on the system. Studies in this emerging field have demonstrated that exposure to lead causes alterations in gut microbiome diversity and metabolic functions. These changes are responsible for the development of obesity when exposure to lead occurs in early childhood. Some of these changes are mediated through epigenetics.
On the other hand, there is some evidence that preexisting depletions in the diversity and quantity of the gut microbiome increases the risk for lead toxicity. This may be independent or further complicated by the impact of dysbiosis on digestion, especially on calcium and iron absorption.
Neutralizing lead exposure
There are medications that are used to chelate lead including calcium disodium edetate (EDTA) which can be used for assessing body stores of lead and for treatment. Interestingly, calcium channel blockers such as verapamil have been found to protect the kidneys against the effects of lead toxicity.
Curcumin has been found to protect kidney cells from the oxidative stress of lead toxicity.
In addition, baicalin, a traditional Chinese herb, has been found to protect the kidneys in a similar manner.
Therapeutic treatment of iron or calcium deficiency can also be effective in reducing lead absorption. Therefore, monitoring mineral levels and supplementation might be a useful approach in management of these cases.
Protecting the body from lead
There are ways to protect the body from the negative effects of heavy metals, including lead. Incorporating therapeutic foods on a regular basis such as cilantro, blueberries, garlic, ginger, onion, green tea, and tomato supplies the body with vitamins, minerals, and nutrients that decrease the risk of lead toxicity. Certain essential metals and vitamins (zinc, calcium, iron, selenium, magnesium, vitamin C, B1, and B6) may be supplemented to provide protection and boost antioxidant levels. Other practices that help the body with detox are exercise, sweating, dry brushing, drinking enough water, and lymphatic massage. Correcting nutrient deficiencies through food and supplements, as well as incorporating routine detox practices, is key to prevent lead absorption and safely remove it from the body, especially in those at high-risk of exposure.
The Bottom Line
Lead exposure remains a global public health issue. Even low levels of lead exposure have been associated with decreased kidney function. In addition to addressing possible sources of exposure, an integrative medicine approach to lead toxicity should use appropriate nutritional evaluations and interventions, as well as biotransformation and antioxidant support to help decrease the impact of toxicity and the risk of chronic kidney disease.
February Research and News
Modifiable Lifestyle Factors for Primary Prevention of CKD: A Systematic Review and Meta-Analysis
Short or Long Sleep Duration and CKD: A Mendelian Randomization Study
People with short sleep duration of less than 6 hours were found to affect kidney function. Since the average person spends a third of her life sleeping this has a great implication on the development and progression of CKD. We wrote a blog about sleep and CKD also.
Read the study.
Particulate Air Pollution and Progression to Kidney Failure With Replacement Therapy: An Advanced CKD Registry–Based Cohort Study in Taiwan
This study showed that air pollution is associated with faster progression of CKD toward failure.
Read the study.
Glycemic Status, Insulin Resistance, and the Risk of Nephrolithiasis: A Cohort Study
This study showed a strong link between insulin resistance and the development of kidney stones specially men even if blood sugar is normal. This is another aspect that we need to pay attention to in our integrative approach to kidney stones.
Read the study.
Genetics of Chronic Kidney Disease
Perhaps the most significant advance in kidney disease in the past few years was the use of genome-wide association studies (GWAS) in various kidney disorders. In these types of studies, various kidney diseases were linked to certain variants in the genetic code. This blog is an introduction into a series of blogs discussing the genetics of chronic kidney disease (CKD).
The Basics
As you may remember, each person inherits 46 chromosomes, 23 from each parent. These chromosomes house the genetic code that determine the characteristics of each person. They are composed of DNA which is a long and windy spiral made up of millions of nucleotides. There are 4 nucleotide bases (adenine, cytosine, guanine, thymine), the sequence of which determines how genetic traits are expressed.
This nucleotide sequence forms the foundation of about 22,000 genes. Our cells read the genetic sequence and use it to make thousands of proteins which are essential for carrying out biological functions that maintain life. In other words, you can think of the cells as protein factories. The DNA code provides the blueprint for all the workers to create proteins. These proteins can function as enzymes, receptors, or other structures that are important for sustaining life.
Errors in the code
Alterations in the sequence of nucleotide bases cause errors in the code and affect the efficiency of the protein production process. These may sometimes lead to disease or increased risk for disease. Interestingly, on occasion they may have no effect on risk and may even be protective against disease. There are three major changes that can occur in the code:
- Single-nucleotide substitution: this is also called single-nucleotide polymorphism or SNPs. These are common.
- Insertion or deletions (indels): a small stretch of DNA can be inserted or deleted from the code.
- Structural variation: a large-scale change or rearrangement in the DNA.
It is fascinating that a single variation in one nucleotide among the set of 3 billion in our DNA can sometimes cause severe and deadly disease and other times have no effect whatsoever.
Why Genetics Are Important?
Genetics play an essential role in many functions in the human body. Genetic changes can affect the type of food that a person prefers to eat. For example, genetic variants in bitter taste receptors in the tongue have been associated with decreased intake of vegetables and increased obesity. Genetic changes can also affect digestion, and absorption of food. They can modify the way we metabolize drugs, and toxins. They can alter the function of vitamins and other nutrients and their interactions.
The Genetics of Disease Risk
For a long time, scientists have been trying to study how variations in genes lead to diseases. Past efforts focused on identifying inherited diseases in the so-called Mendelian patterns. However, recent advances in GWAS studies are making it more possible to identify genetic changes that can be associated with increased risk of a disease. Genetic variations in one or several genes can lead to minor changes that collectively may increase the susceptibility for certain common diseases such as diabetes and high blood pressure. This is an evolving field of study and we will continue to learn about it every day.
Genetics of Kidney Disease
There are well-known and relatively common genetic disorders such polycystic kidney disease, Alport’s Syndrome and Fabry’s disease. GWAS studies has identified more than 500 genes that are associated with kidney diseases. Current evidence suggests that a significant genetic component plays a role in the development of kidney disease. These are evident from the ethnic variabilities in certain diseases such as the higher incidence of IgA nephropathy among Asians.
Genetics can also determine the severity of a kidney disease, the age of onset and the likelihood of ending up with End-Stage Kidney Disease. For example, different gene mutations that can cause polycystic kidney disease can have different outcomes.
In addition, genetic variations can explain the differences in susceptibility of the kidneys to systemic disease such as diabetes and lupus. These variations may explain why some people with diabetes get severe kidney disease while others don’t.
Finally, alterations in several genes have been associated with increased risk for CKD. There are several candidate genes. Mutations in the UMOD that encodes uromodulin is one of these candidates. Uromodulin, which used to be called Tamm-Horsfall protein, is excreted in certain portions of tubules and protects from urinary tract infections. The Shroom3 gene is another candidate that has been associated with increased risk for CKD.
Next generation sequencing
Various methods for identifying genetic variants have been used in the past. Next generation sequencing is a state-of-the-art technology that allows DNA sequencing of the entire human genome within a single day. It also captures a broader spectrum of variations that can affect the genetic code. This revolutionary technology is now available for kidney disease patients and can identify various mutations that are associated with CKD or can increase the risk or severity of CKD.
The Bottom Line
Genetics is one of the factors that lead to the development of kidney disease. For genes and their variants to contribute to a disease, we need to understand all of the factors that explain why people with the same variant may have different outcomes. Understanding the interaction between genetics, epigenetics and environment are crucial in this regard. Ongoing research in this field will not only increase our understanding of kidney disease risk but also our ability to find various lifestyle modifications and therapies that can help decrease the burden of CKD worldwide.
Professionals: You can get free CME and learn more about the genetics of kidney disease here.
The Gut-Derived Uremic Toxins
The kidneys are considered the filtration system for various byproducts and toxins. Chronic kidney disease (CKD) is associated with accumulation of waste products due to decreased clearance by the failing kidneys. These “uremic toxins” come from multiple sources.
Recently, there has been a growing interest in the gut-kidney axis, which refers to the overlapping relationship between gut integrity, microbiome diversity, resulting inflammatory process and kidney disease. The interaction between the gut and kidneys is very complex and can be divided into two major categories: the gut-derived uremic toxins that can worsen kidney disease, and the inflammatory autoimmune response that can trigger kidney disease. In this blog, we will focus on the gut-derived uremic toxins.
Uremic Retention Molecules (URMs)
Uremic toxins, accumulated waste products due to decreased renal clearance, have been linked to systemic inflammation. Recently, researchers have been successful at identifying many of these molecules thanks to a technique called metabolomic analysis. In general, URMs are divided into three categories:
- Small water-soluble molecules
- Middle size molecules
- Protein-bound uremic molecules
However, for our purpose, it is more helpful to divide these toxins according to their origin:
- Endogenous uremic toxins: toxins or waste that naturally and normally occur during metabolism or catabolism
- Exogenous uremic toxins: related to dietary intake
- Gut-derived uremic toxins: generated by pathogenic gut microbiota in the presence of dysbiosis
Gut-derived uremic toxins
The human gut contains trillions of microorganisms, collectively referred to as the gut microbiota or microbiome . The composition of the gut microbiome varies from person to person due to genetics, environmental factors, dietary, and disease state. These microorganisms are in constant communication with the body. They also produce multiple metabolites, nutrients, and cell signals for various physiological functions. [read more about the microbiome here]
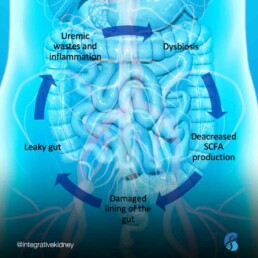
Dysbiosis describes a state where “bad” bacteria (or other organisms like yeast or parasites) outgrow the “good” bacteria. In dysbiosis, we see a rise in inflammatory markers that interact with the lining of the gut and result in damage and increased intestinal permeability (“leaky gut”). This causes shifts in the breakdown of nutrients, including amino acids, which leads to the formation of many gut-derived uremic toxins.
Thanks to recent advances in metabolomic analysis, researchers have identified more than 60 uremic toxins that are derived from the gut. A number of studies have demonstrated that URMs are associated with high cardiovascular burden and mortality in CKD. There are four major gut-derived uremic toxins associated with CKD: Indoxyl sulfate (IS), para-Cresyl sulfate (pCS), Trimethylamine-N-oxide (TMAO), and Indole acetic acid (IAA).
Indoxyl sulfate (IS)
IS is a protein-bound uremic toxin. Gut microbiota produce indole from the breakdown of dietary tryptophan, which is then further metabolized to IS in the liver. The majority of IS circulates bound to albumin in the blood. It is excreted by the kidney tubules through organic anion transporters.
IS is one of the most studied uremic toxins. It has been associated with vascular calcifications and increased risk of death from heart disease in patients with advanced kidney disease. Higher levels of circulating IS have also been associated with faster progression of CKD.
Para-Cresyl sulfate (pCS)
This is another protein-bound uremic toxin that is produced by the metabolism of amino acids tyrosine and phenylalanine by intestinal bacteria. This leads to the formation of p-cresol which is metabolized in the liver to pCS. pCS also circulates in the blood bound to albumin and is excreted by the kidneys in the same mechanism as IS.
Serum pCS levels increase with worsening kidney function. Similarly to IS, pCS was also found to predict the progression of kidney disease and is associated with an increased risk of death from heart disease in kidney disease patients.
Trimethylamine-N-oxide (TMAO)
TMAO is one of the small water-soluble URMs. It is produced by the metabolism of dietary L-carnitine and choline. The latter two compounds are metabolized into trimethylamine by gut bacteria, which is then absorbed by the gut and oxidized in the liver to form TMAO. The kidneys are the primary source for eliminating TMAO from the body. Dysbiosis and decreased kidney function have been documented to lead to elevated levels of TMAO.
TMAO levels increase with worsening kidney function and are associated with increased progression of kidney disease, increased risk of heart disease and all-cause mortality in kidney patients.
The L-carnitine controversy
L-carnitine is a relatively small water-soluble compound that is found abundantly in food sources including in red meat, dairy, poultry, and fish. Lysine, methionine, ascorbate, niacin, pyridoxine, and iron are among the major sources of endogenous carnitine production.
There is evidence that patients with kidney disease are deficient in L-carnitine and that supplementation leads to improved outcomes. However, there’s seemingly contradictory evidence that, the metabolism of L-carnitine by gut bacteria leads to the production of TMAO which, as described above, is associated with increased mortality in kidney patients.
One explanation for this discrepancy is that research on L-carnitine often fails to distinguish CKD patients who are not on dialysis from those on dialysis. In fact, one study showed L-carnitine levels are actually high in patients with CKD but are low in dialysis patients.
Another reason for the confusion is that most research on L-carnitine supplementation uses intravenous L-carnitine, which bypasses the gut microbiota and liver and, therefore, may not lead to the production of TMAO. Another important consideration is the composition of the microbiome and the intestinal integrity of the gut. Those with certain dysbiotic markers might be at higher risk for producing TMAO and failing to break it down sufficiently, leading to increased kidney injury.
The literature seems to support the need for restricting oral intake of dietary or supplement sources of L-carnitine in patients with kidney disease who are not on dialysis. This might also explain why CKD patients benefit from diets that are dairy-free and low in red meat.
Indole acetic acid (IAA)
IAA is yet another protein-bound uremic toxin that results from the metabolism of tryptophan by gut bacteria. Levels of IAA are proportional with CKD stage progression and normalize after kidney transplantation. IAA is linked to vascular inflammation and oxidative stress and has been associated with mortality and increased risk of death from heart disease in patients with CKD.
The role of diet
Dietary habits play an important role in gut microbiota composition. Diets high in animal protein are associated with increased dysbiosis and intestinal hyperpermeability and also contain a higher amount of amino acids precursors of uremic toxins. Meanwhile, whole-food and fiber-dense plant-based diets are associated with the growth of healthy gut microbiota, leading to decreased inflammation and better intestinal integrity.
Levels of protein intake have been studied in relation to CKD. Interestingly, it is not the amount but the type of protein that is associated with kidney disease. In fact, a large study of 12,000 adults with normal kidney function found no significant association between total protein intake and the incidence of CKD. The same study found that individuals consuming more red and processed meat were at higher risk for CKD compared to those consuming more vegetable protein. Red meat intake has also been reported to increase the risk of worsening kidney function requiring dialysis.
There are various additional factors that should be considered as well, including consumption of processed carbohydrates, simple sugars, and reduced consumption of whole fruits, vegetables, and other fiber sources like whole grains. These nutrient-poor dietary habits can lead to dysbiosis which in turn produce inflammatory molecules that can lead to kidney disease. This can cause further accumulation of inflammatory uremic toxins.
Furthermore, the worsening of kidney function and accumulation of URMs can lead to further dysbiosis, and so on, the cycle repeats. This is what we describe as the dysbiosis cycle in kidney disease.
To date, unfortunately, there are no studies that measure the effectiveness of dietary interventions on URM levels in kidney disease and subsequent prognosis. Studies focusing on the use of prebiotics and probiotics to improve gut health and reduce inflammation and CKD risk have had mixed results. However, a major weakness is that these studies only look at a single intervention that impacts kidney disease when, in reality, the process is much more complex. Future studies should take into consideration the complex interplay of the dietary and lifestyle factors on GI integrity, microbiome balance, genetics, and kidney outcomes.
The bottom line
There is a significant link between the microbiome, gut integrity, genetics, diet, lifestyle, and kidney disease. Most of this can be traced back to the accumulation of URMs in CKD causing a vicious cycle. The conventional approaches to improve gut health and slow the progression of CKD have yielded mixed results.
Casting a wider net to include considerations for all factors that contribute to gut integrity, inflammation, and CKD risk must also be examined, including environmental exposures, genetic risk factors, metabolic factors and changes in body fluid volume, and overlapping factors that contribute to diabetes and hypertension, collectively contribute to increased CKD risk. Hence, we advocate for a comprehensive approach that starts with a gut restoration protocol and addresses the above factors in patients with CKD.
Implementing Lifestyle Modifications for Kidney Health
Lifestyle modifications are crucial in our integrative approach to kidney health. They are one of the major differences between the conventional and integrative/Functional Medicine approach to halting disease progress. Let’s discuss some of the challenges of adopting lifestyle changes and provide practical tips to help with successful implementation.
As mentioned in a previous blog, the integrative approach to many kidney diseases should be personalized, yet comprehensive. It starts with understanding an individual’s genetic predisposition, current and previous lifestyle choices and exposures, triggers of the current disease process, and unique nutritional status. In addition, it’s important to identify an individual’s spiritual, mental and emotional state. This type of approach can help the provider develop a personalized plan that includes attainable lifestyle modifications for the patient.
The ability of the patient to make these changes is the cornerstone of this tailored management plan. Although implementing and adhering to lifestyle modifications can be challenging, it’s not a reason to quit. In order to understand how to overcome these challenges, we talked about the brain and implementing changes in this blog. Understanding synaptic pruning and neuroplasticity can help us in this process.
Implementing Lifestyle Modifications
Now let’s utilize what we discussed above to help patients implement the lifestyle modifications we recommend for those with kidney disease. Of course, willpower varies from person to person. So, what we are suggesting here might not be for everyone, but we think the information is helpful.
Think about it
Now that we know that the brain is hardwired to follow habits, we can approach lifestyle habits without judging or beating ourselves up. The first step in implementing lifestyle modifications is to think about these changes. It will help to write down or talk to someone about the thoughts associated with these changes. Think about lifestyle habits that are currently working and any that are not in order to help identify patterns. Identifying these patterns can be a good starting point to help the brain perceive the need for change. Remember, the brain wants to be efficient and it will always default to the “pre-wired” unhealthy habits if we let it. So, spending extra energy and utilizing that pre-frontal cortex to teach the brain new things is required in the beginning.
Pick one thing
Once patterns have been identified, it’s best to implement one change at a time to avoid overwhelming the brain. It can be helpful to work with a provider or coach to devise a plan of action. Oftentimes, it is beneficial to start with easier changes, as successful implementation of the new habits can help build momentum. For example, an individual may decide an easy change would be to not snack or drink sugary beverages/alcohol after dinner. It is important to note that what is easy for one person may not be as easy for another, so personalization of an action plan is critical.
Plan it
Having a plan can make or break a person’s ability to stick with the new habits. It’s easy for the brain to default to old habits when there isn’t a plan in place. For example, using the weekend to plan out and/or prepare meals for the upcoming week can help ensure healthy eating habits during the week for someone who orders take-out when they get home tired after work. Taking the guess work out of things can set someone up for success.
Learn from mistakes
Let’s face it, mistakes can happen. They don’t indicate failure or give reason to quit. There may have been some variables that were not accounted for. It’s important to learn from mistakes and improve the planning process for the future.
Repeat, repeat, repeat
The most important step in making these modifications permanent is repetition. Repetition creates new patterns and neural pathways via synaptic pruning so that the brain will default to them instead of the previous unhealthy habits. The amount of time this takes depends on the individual’s willpower and commitment. This is not going to be easy but, as they say practice makes perfect.
The Bottom Line
Implementing lifestyle modifications is crucial in the fight against the chronic kidney disease epidemic and foundational to the integrative approach to kidney health. It can be a challenging process but understanding the brain’s efficacy and patterns can help individuals make the changes needed to lead a healthier lifestyle.
The Brain And Lifestyle Changes Implementation
Lifestyle modifications are crucial in our integrative approach to kidney health. They are one of the major differences between the conventional and integrative/Functional Medicine approach to halting disease progress. Let’s discuss some of the challenges of adopting lifestyle changes and provide practical tips to help with successful implementation. In this blog, we will talk about some of the basics of physiology and psychology to understand how the brain can implement lifestyle changes. We will discuss the brain and lifestyle changes.
The Efficient Brain
Despite the fact that the brain utilizes approximately 22% of the body’s energy needs, it is quite efficient in many of its processes. The major cells in the brain, called neurons, connect via synapses. For the purpose of this blog, it is easier to think about the brain from an evolutionary standpoint. By doing that, we can divide the brain into the primitive brain (limbic brain and basal ganglia) and the advanced brain (pre-frontal cortex). The primitive brain processes emotion, behavior, motivation, and memory in addition to posture and voluntary movement, while the pre-frontal cortex is involved in critical thinking and learning.
In essence, during the process of learning, the pre-frontal cortex develops the necessary thinking and process to learn a task. After this learning is established and practiced, it becomes automatic and processed at the level of the “primitive brain” without further energy expenditure from the pre-frontal cortex.
Take learning how to drive for example. Initially, it takes a lot of processing to learn to coordinate all the different sensory and motor functions that are involved. But after a few months of driving all these skills become automatic and processed without further thinking or involvement from the pre-frontal cortex.
Synaptic Pruning
The process of coordinating different sensory and motor functions required to accomplish certain tasks involves what is called synaptic pruning. In this process, the brain coordinates the action of several neurons and makes them “fire” together to accomplish these functions leading to a specific task. Initially, as the pre-frontal cortex is learning, the process can be clumsy. It is as if the brain is forging a new path between trees in a forest. As the brain gets better at this coordination, the process becomes faster and more automated. In fact, it becomes so fast it’s as if the brain converted that path in the forest into a highway. This is the basis of the quote: ‘neurons that fire together, wire together’. Basically, the brain forms “neural pathways” for different tasks. Synaptic pruning is essential for brain development and learning. However, recently it has been implicated in several disease processes.
One important aspect of synaptic pruning is the development of habits. Some can be survival habits, some can be healthy habits, yet some can be unhealthy. Unhealthy habits include eating highly-processed, sugar-laden food when stressed or depressed, drinking one or more alcoholic beverages after work to cope with stress, overeating to hide emotions, and cigarette smoking and other addictions. In other words, the brain develops a highway that starts with a negative emotion and ends with an unhealthy habit.
Neuroplasticity
The good news is that the brain can change these unhealthy neural pathways and create new ones in a process called neuroplasticity. Neuroplasticity is the capacity of the brain cells to change in response to internal and external factors. The brain has the ability to forge new pathways and learn new things at any point in time. In fact, it has been found that challenging the brain can even lead to neurogenesis, the formation of new brain cells. When it comes to the brain, age is just a number. Neuroplasticity has been documented in 89-year-old people!
In short, you can teach your brain new habits, new skills, and new knowledge at any point in life. Of course, exercise, a healthy diet, stress reduction techniques and good sleep habits can improve the ability of the brain to learn these new skills and habits. But, what we want to discuss is how to utilize neuroplasticity in developing new healthy choices to implement healthy lifestyle changes. So, let's read about that in this blog.
Very Low Protein Diet for Kidney Health
High intake of animal protein has been associated with an accelerated decline in kidney function and increased protein being spilled in the urine. However, there is a generalized fear of recommending a very low protein diet to patients with kidney disease. There is concern this diet may provoke malnutrition especially because poor nutritional status could increase risk of illness and even death in these patients. Let’s review the premise of a very low protein diet for kidney health and how it can be properly used for patients with kidney disease.
We discussed the effect high intake of animal protein has on kidney health in a previous blog. According to the National Institute of Health, the Dietary Reference Intake (DRI) of protein for the average person is 0.8-1.6 g/kg/day (or 0.36-0.75 g/lb/day). However, according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, kidney patients should limit their protein intake. A low protein diet (LPD) by the KDIGO guidelines is 0.8 g/kg/day (or 0.36 g/lb/day). That means a kidney patient who weighs 150 lbs (68 kg) has a dietary protein target of approximately 54 grams of protein per day (0.8g/kg x 68kg). Furthermore, it’s suggested that at least half of that comes from plant-based sources. Protein intake, along with other related nutrients, should be tracked closely through nutrient intake analysis, ideally by a nutritionist.
Different dietary protein regimens have been proposed in the literature for patients with Chronic Kidney Disease (CKD):
- A low-protein diet (LPD), with protein target of 0.6-0.8 g/kg/day;
- A very low-protein diet (VLPD) (0.3 g/kg/day) supplemented with essential amino acids, or
- A very low-protein diet (VLPD) (0.3 g/kg/day) supplemented with nitrogen-free keto-analogues of essential amino acids.
The controversy in the literature, however, has always been whether or not lowering the targeted protein intake puts patients at risk for nutritional deficiency, including essential amino acids and other nutrients often found in high protein foods like folate, B12, carnitine, and zinc. And in that case, is supplementation among these patients necessary and useful to maintain adequate nutrition?
Let’s focus on amino acids, but first let’s review.
What are essential amino acids?
Amino acids (AA) are the basic organic building blocks of protein. There are a total of twenty-two AA, each composed of a core structure made of nitrogen, carbon, hydrogen and oxygen, and variable side chain groups unique to each AA.
Of the twenty-two AA, there are nine essential amino acids (EAA). They’re called “essential” because the human body cannot make them, and therefore we must get from our diet. These EAA are:
- Histidine
- Leucine
- Isoleucine
- Valine
- Lysine
- Threonine
- Methionine
- Phenylalanine
- Tryptophan
The remaining thirteen AA are termed “unessential” not because they’re not important, but because the body can make them through various biochemical processes as long as the necessary building blocks are available from the diet.
When restricting protein intake in kidney patients, it is important that the patient receive adequate intake of EAA since adequate levels of these essential nutrients can only be maintained through food. This can be challenging to keep balanced, which is why we suggest working with a nutritionist to track and support adequate intake.
Using ketoacid analogues with very low protein diet for kidney health
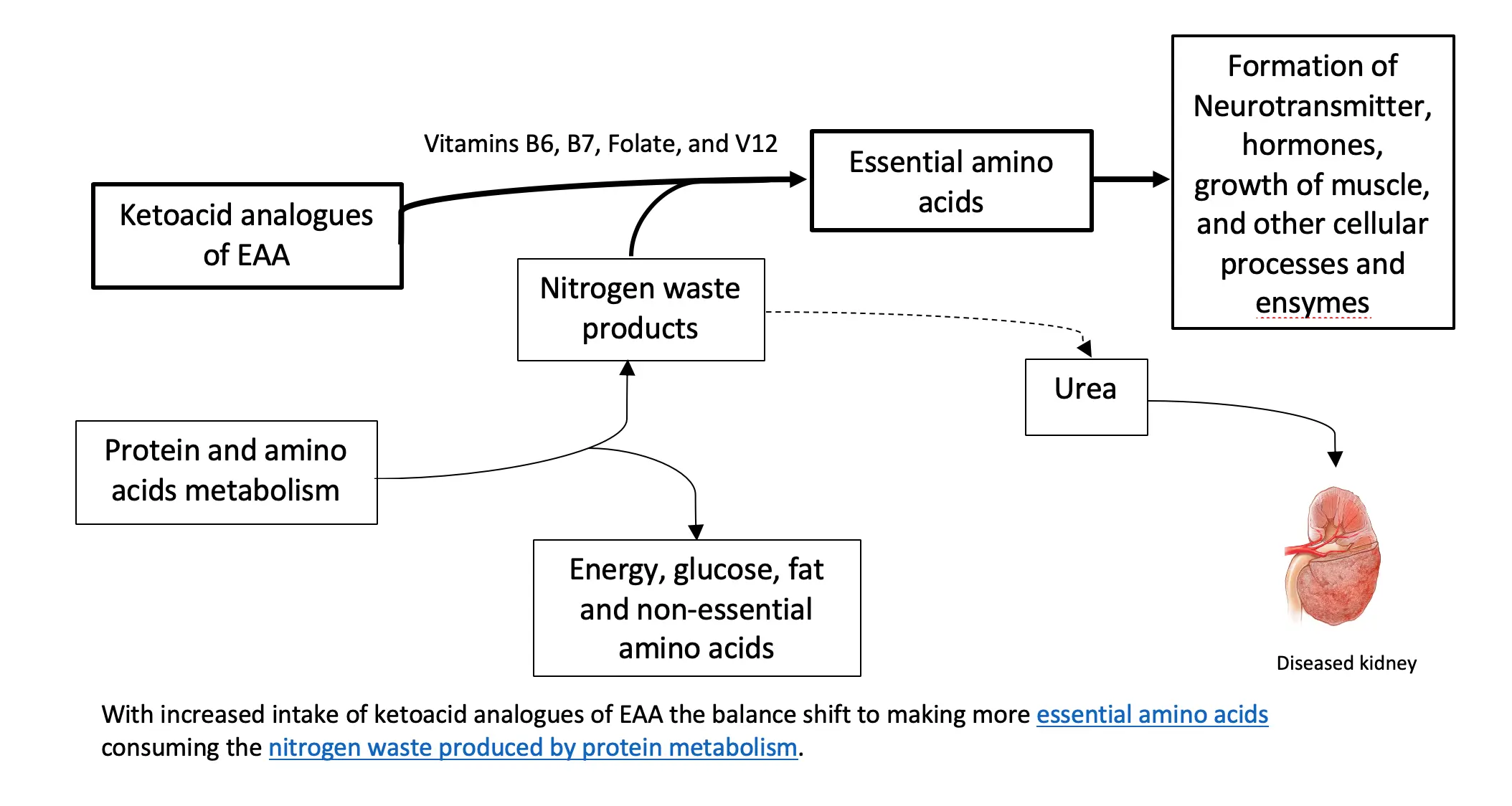
To understand the relevance of ketoacid analogues in CKD, we need a biochemistry lesson in AA metabolism. Biochemistry isn’t everyone’s thing, so we summarized it here.
One thing to remember about AA metabolism is that it requires cofactors which are nutrients that facilitate the reactions. Vitamin B6 is a major cofactor here. Other cofactors needed in the amino acid breakdown (catabolism) include biotin, folate, and B12.
How do ketoacid-analogue supplements help in kidney disease?
Theoretically, the process described above can be used in reverse to reduce circulating nitrogen and the formation of nitrogen waste products (i.e., like urea), and, therefore, decrease the burden on the diseased kidneys. Supplementing ketoacid analogues of EAA in kidney patients on a LPD and VLPD, might simultaneously achieve two goals: 1. It closes the gap on adequate EAA nutritional need 2. It decreases the toxic nitrogen waste products contributing to disease complications. There is also some evidence that certain analogues can decrease protein breakdown in the body.
Some studies have demonstrated that very low protein diets supplemented with ketoacid analogues decrease waste product generation in kidney disease and prevent protein-energy decline. Supplementation of keto-analogues in kidney patients was found to decrease protein in the urine as well as improve bone health, insulin sensitivity, and blood pressure control. In addition, these dietary protein restrictions with keto-analogue supplements have been found to delay symptoms of kidney failure and the need for dialysis.
However, one of the major concerns of supplementing ketoacids in kidney patients on VLPD is that it does not address the other micronutrients and cofactors that need to be supplemented. it also does not take into account the carbohydrate and healthy fat content of a healthy diet.
Ketoacid analogue preparations
There are a few different preparations available in Europe, but only one available in the US. If one looks at the content of these preparations, it will become obvious that the kidney patient will need several pills of ketoacid daily to achieve an adequate daily dose. This makes this therapy very expensive and impracticable.
Luckily, there’s a way to use diet to balance macronutrients to induce the same effect. A low protein diet, with the majority of protein coming from whole food plant sources. When consuming animal protein, we recommend organic and grass-fed sources. Strictly vegetarian patients can still get adequate sources of EAA if they plan their diet well with the help of a nutritionist. If AA supplements are determined to be needed, one should aim to achieve the recommended daily intake from a trusted source (with good quality control). According to the World Health Organization, the recommended daily dose of each of the EAA is as follows:
| Essential Amino Acid | Recommended daily intake |
| Lysine | 30 mg/kg/day |
| Leucine | 39 mg/kg/day |
| Isoleucine | 20 mg/kg/day |
| Valine | 26 mg/kg/day |
| Threonine | 15 mg/kg/day |
| Phenylalanine | 25 mg/kg/day |
| Tryptophan | 4 mg/kg/day |
| Histidine | 10 mg/kg/day |
| Methionine | 10 mg/kg/day |
The bottom line on very low protein diet for kidney health
High animal protein intake is associated with faster progression of CKD, likely due to excess production of nitrogen byproducts from protein breakdown. Following a whole food plant-dominant diet can be helpful to delay the progression of kidney disease. The use of ketoacid analogues as supplements may not be practical. In order to assure that the patient is getting adequate sources of EAA and other necessary nutrients, dietary restrictions should be implemented with the help of an experienced nutritionist, preferably one trained in a personalized approach to kidney health.
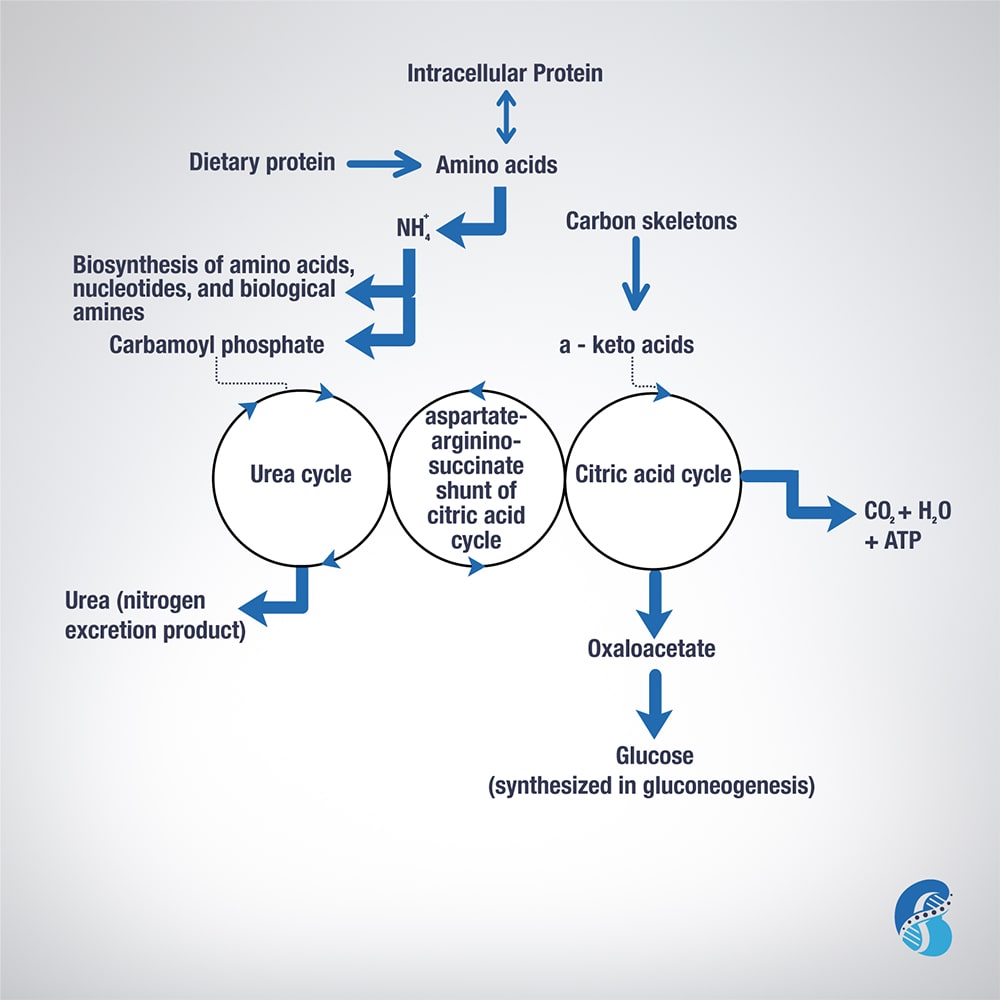
Biochemistry of Amino Acids Metabolism
To understand the relevance of ketoacid analogues in chronic kidney disease, one need to learn the biochemistry of amino acids (AA) metabolism. This blog will attempt to describe it in short.
Excess AA in a protein-rich diet is metabolized through a process called transamination. This involves the removal of the nitrogen (or the amino group NH2 side chain) and replacing it with a keto group carbon structure forming a ketoacid or ketoacid analogue.
Apart from lysine and threonine, every EAA has a keto-analogue. The ketoacid then undergoes oxidation to carbon dioxide, water, and a three to four-carbon unit that can be converted by the liver into glucose (by gluconeogenesis) or into ketone bodies for energy.
The conversion of an amino acid to ketoacid depends on the enzyme aminotransferase. This enzyme is reversible (meaning it can convert amino acid to ketoacid or a ketoacid to amino acid). The speed and direction of this conversion depends on the amount of amino acids available, the amount of keto-acids formed, and the availability of the cofactors needed to power the process. These cofactors are nutrients that facilitate the reactions. Vitamin B6 is a major cofactor for this enzyme. Other cofactors needed in the amino acid breakdown (catabolism) include biotin, folate, and B12.
Now, remember that amino group (-NH2)? That can be used in the biosynthesis of non-essential AA and the excess ends up metabolized in the urea cycle to form urea and other nitrogen waste products that are excreted by the kidneys. It is worth noting that the AA arginine acts as a regulator of the urea cycle. Remember, arginine is essential for the production of nitric oxide, which plays an important role in blood pressure management. Issues with the urea cycle and nitric oxide balance may explain why kidney patients have blood pressure problems and may not feel well with arginine supplementation.
Overall, this process is summarized in this figure.
Genetics of Kidney Stones
Kidney stone development is the composite end-result of multiple factors including genetic, dietary and environmental, and can have serious consequences. They are often painful and if left unaddressed can lead to more severe conditions such as obstruction of urinary flow and permanent damage to the kidneys. Recurrent kidney stones are one of the common causes of CKD. Here we will discuss the genetics of kidney stones.
In this series we’re focusing on the integrative approach to preventing kidney stone formation.
Conventional approaches to kidney stones tend to focus on medications, surgical removal, and using ultrasonic waves to break up stone. It rarely approaches the root cause including risk factors to prevent stone formation.
We covered the impact of diet, the microbiome and gut health, and electrolyte imbalances on kidney stones in previous blogs. Here we will discuss the role genetics play in kidney stone formation.
The Genetics of Kidney Stones
There seems to be a familial link when it comes to the development of kidney stones. In fact, two thirds of patients with calcium-containing kidney stones have a relative with kidney stones. Recently, genome-wide association studies uncovered several genetic sequence variants (SNPs) that lead to increased risk of kidney stone development. Although we are still scratching the surface in understanding the contribution of genetic factors to stone formation, we do know that we can modulate these risks through environmental and dietary modifications.
Genetic Variations in Calcium Handling
In a previous blog, we discussed the role of the kidney filtration units (specifically the nephrons). The kidneys are responsible for filtering large volumes of blood daily. This function is crucial, and, the unique design of the nephrons make it able to adjust this filtrate and prevent dehydration. This intricate design also makes the kidneys crucial in the balance of water and many electrolytes.
Calcium is one of the electrolytes filtered and reabsorbed in the kidneys. Calcium sensing receptors (CaSR) are present in kidney cells and are essential for the reabsorption of calcium. These receptors increase and decrease the amount of calcium reabsorbed based on the calcium level in the blood. In other words, activating these receptors increases the amount of calcium lost in the urine.
Single nucleotide polymorphisms (SNPs) in CaSR have been found to alter the function of these receptors leading to increased urinary excretion of calcium. Of particular interest, SNPs in rs7652589 and rs1501899 were associated with kidney stones in patients with normal citrate excretion. SNPS in rs1801725, rs1042636 of the CaSR gene were also associated with kidney stones in various populations.
Another gene that has been associated with increased calcium in the urine is the gene coding for the protein Claudin-14, responsible for forming tight junctions. This protein helps connect adjacent cells to form a barrier which acts like a gate, separating blood from urine. Tight junctions are also responsible for ensuring that minerals don’t pass between the cells. SNPs in the genes that code Claudin-14 (CLDN14) alters the integrity of these “gates” and allows for calcium to “sneak” between cells into the urine, increasing the risk for kidney stone formation. Specifically, SNPs at the locations rs219778, and rs219780 of the CLDN14 gene were significantly associated with kidney stones.
Genetic Variations in Vitamin D Receptors (VDR)
Vitamin D plays a crucial role in calcium balance. Studies have shown that vitamin D increases the absorption of calcium from the gut and also increases calcium excretion in the urine. Vitamin D receptors are essential for vitamin D to exert its action on calcium balance.
Some mutations or SNPs in the VDR are associated with increased absorption and excretion of calcium, significantly increasing the risk of kidney stones. It is worth mentioning here that there is some controversy about the link between vitamin D supplementation and kidney stone formation. Vitamin D deficiency appears to be common among kidney stone formers. This is likely because low vitamin D causes calcium loss from the bone in order to maintain normal calcium range in the blood for cardiovascular protection. Even though vitamin D3 supplementation may increase calcium excretion in the urine, it has not been conclusively found to increase the risk of kidney stone formation.
Therefore, genetic assessment may be a key to identify patients who are at risk of kidney stone formation from taking vitamin D supplements.
Genetic Variations in the Handling of Other Minerals
The kidneys are crucial for balancing many minerals in our bodies such as magnesium, phosphate, oxalate and others. Genetic mutation or SNPs affecting the genes that code for the channels or receptors that regulate these minerals can also impact the risk of kidney stones. Some SNPs on the other hand can be protective against kidney stones such as SNPs in the UMOD gene. Discussion of this long list of SNPs requires details beyond the scope of this blog, but we summarized most of the genes that have been associated with kidney stones in the table below.
| Gene symbol | Gene name | Phenotype |
| ADCT10 | Adenylate cyclase 10 | Increased calcium excretion |
| AGXT | Alanine-glyoxylate aminotransferase | Increased oxalate excretion |
| CA2 | Carbonic anhydrase II | Osteoporosis + decreased acid excretion |
| CASR | Calcium-sensing receptor | Increased calcium excretion |
| CLCN5 | Chloride channel, voltage-sensitive 5 | Dent disease |
| CLCNKB | Chloride channel, voltage-sensitive Kb | Bartter Syndrome, type 3 |
| CLDN14 | Claudin 14 | Increased calcium excretion |
| CLDN16 | Claudin 16 | Increased calcium and magnesium excretion |
| CLDN19 | Claudin 19 | Increased calcium and magnesium excretion |
| CYP24A1 | Cytochrome P450 | Decreased breakdown of vitamin D3 |
| GRHPR | Glyoxylate reductase | Increased oxalate excretion |
| HOGA1 | 4-Hydroxy-2-oxoglutarate aldolase 1 | Increased oxalate excretion |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 | Increased uric acid excretion |
| SLC12A1 | Solute carrier family 12, member 1 | Bartter syndrome, type 1 |
| SLC26A1 | Solute carrier family 26, member 1 | Calcium oxalate kidney stones |
| SLC22A12 | Solute carrier family 22, member 12 | Decrease uric acid excretion |
| SLC2A9 | Solute carrier family 2, member 9 | Decreased uric acid excretion |
| SLC34A1 | Solute carrier family 34, member 1 | Calcium phosphate kidney stones |
| SLC34A3 | Solute carrier family 34, member 3 | Calcium phosphate kidney stones |
| SCL3A1 | Solute carrier family 3, member 1 | Increased Cystine excretion |
| SLC4A1 | Solute carrier family 4, member 1 | Decrease acid excretion (dRTA) |
| SLC7A9 | Solute carrier family 7, member 9 | Increased Cystine excretion |
| SLC9A3R1 | Solute carrier family 9, subfamily A, member 3, regulator 1 | Calcium phosphate kidney stones |
| UMOD | Uromodulin (most common urine protein) | Protective against kidney stones |
| VDR | Vitamin D (1,25-dihydroxy D3) receptor | Increased calcium excretion |
| XDH | Xanthine dehydrogenase | Increased xanthine excretion |
The Bottom Line
There are many factors that impact the risk of kidney stone development. Although there are pure genetic diseases that are associated with kidney stones, often the increased risk is subtle or offset by other factors. Increased risk, when combined with other factors including nutrient depletion, dysbiosis, electrolyte imbalances and dehydration, may lead to the development of kidney stones in some. Assessing the genetic profile of kidney stone patients can help identify the root cause of the disease to tailor appropriate, personalized management. Practitioners working with individuals to prevent kidney stone formation should formulate a comprehensive and individualized intervention that modifies all relevant components in their integrative approach.
Electrolyte Imbalances and Kidney Stone Formation
The formation of kidney stones is complex, with influence from multiple factors including genetic and environment. Stones can be costly. Not only can they be painful, if left unaddressed, more serious conditions can arise such as obstruction and permanent damage to the kidneys. In this blog we focus on electrolyte imbalances and kidney stone formation.
As discussed in a previous blog, there are five major types of kidney stones: calcium oxalate, calcium phosphate, uric acid, struvite (magnesium ammonium phosphate), and cystine. Calcium oxalate is by far the most common, comprising approximately 75% of kidney stones. Therefore, the first step in evaluating a kidney stone patient is identifying the type of kidney stone they are forming. This can be done by stone analysis and by obtaining a 24-hour urine analysis for various metabolites and electrolytes.
In this series, we’re focusing on the integrative approach to preventing kidney stone formation.
Conventional approaches to kidney stones typically focus on medications, surgical removal, and using ultrasonic waves to break up a stone. It rarely addresses the root cause including risk factors to prevent stone formation.
We covered dietary approach and microbiome and gut health in previous blogs. In this blog, we will discuss the impact of electrolyte imbalances on kidney stone formation and prevention.
Calcium imbalances
Since most kidney stones are composed of calcium oxalate, oftentimes patients ask if restriction of dietary calcium is necessary. Dietary calcium intake has many benefits including maintaining adequate bone health and decreasing oxalate absorption from the gut. In fact, evidence suggests that restricting calcium intake leads to increased oxalate absorption, in turn increasing the risk for kidney stone formation. Furthermore, there is a significant link between kidney stones and metabolic bone diseases such as osteoporosis, although it is not yet clear if this link is related to calcium imbalances or other causes.
It’s the concentration of calcium in the urine that is important to track. The higher the urinary concentration of calcium, the higher the risk of oxalate binding to calcium (or other minerals) and forming kidney stones.
There are several factors that lead to increased calcium concentrations in the urine, including increased calcium excretion by the kidneys and decreased water intake. Factors increasing urinary calcium excretion include high sodium (salt) intake, genetic factors, certain medications, and certain medical conditions such as disorders of the parathyroid gland.
This explains why restricting sodium intake and increasing water intake are useful for decreasing the risk of stone formation. It is generally recommended that kidney stone formers drink at least 2 liters of filtered free water every day (some require more, especially if they are overweight/obese or losing fluid from physical activity or sweating). In general, we recommend that patients maintain a balanced diet providing 1000 mg per day of calcium. When taken with meals, calcium supplements bind oxalate in the food and prevent it from getting absorbed; however, calcium supplementation is controversial and should be monitored closely by a provider.
Oxalate imbalances
In a previous blog, we mentioned that oxalates play a role in the formation of kidney stones with 75% of kidney stones a result of calcium oxalate formation. As we discussed above, there is a significant connection between calcium consumption and the level of absorbed oxalate. Low oxalate diets have been proposed as a way to minimize stone formation. However, these are incredibly challenging to maintain. Oxalate restriction is challenging because oxalates are present in a large number of healthy foods, risking nutrient deficiencies. Furthermore, the gut microbiome plays an important role in the absorption of oxalate.
Additionally, there are a few genetic variations in the oxalate gatekeepers in the gut and red blood cell transporters that impact/increase oxalates in the urine. Problems with fat digestion and absorption as seen in small bowel disease, gastric bypass, or pancreatic insufficiency can lead to increased oxalate absorption in the colon. That is why it is important to address gut integrity with a comprehensive gut restoration protocol in patients with kidney stones.
Citrate imbalances
Urinary citrate may be the most significant factor in formation of calcium oxalate stones. Citrate helps increase urinary pH (making it more alkaline or basic), preventing the binding of calcium and oxalate. It also binds calcium in the urine, further preventing kidney stone formation. The concentration of citrate in the urine is dependent on blood acidity, the higher the dietary acid load, the higher the risk of developing kidney stones. This phenomenon may explain why diets high in animal protein have been associated with the development of kidney stones (more about that in another blog here).
In addition, potassium depletion is often associated with pH changes that lead to decreased urinary citrate levels. Potassium depletion can also increase calcium excretion in the urine. Therefore, it is important to correct any potassium depletion in patients with kidney stones. This is why most citrate supplements that are used for kidney stone prevention are usually in the form of potassium citrate.
Magnesium imbalances
Magnesium plays an important role in the prevention of kidney stones. Although the way magnesium decreases the risk of kidney stones is not completely clear, evidence suggests that magnesium lowers oxalate absorption in the gut. Magnesium also increases the level of citrate in the urine which, as we saw above, decreases the risk of kidney stones.
Finally, magnesium can bind oxalate in the urine making it less available for binding with calcium and forming kidney stones. Magnesium oxalate compounds are 100 times more soluble than calcium oxalate. In fact, supplementing magnesium was found to be effective in preventing kidney stones even with patients with normal magnesium.
Join us to end the kidney disease epidemic
Uric acid imbalances
Uric acid stones are common in patients with metabolic syndrome and type 2 Diabetes. Elevated uric acid levels in the urine can lead not only to uric acid stones but also to calcium oxalate stone formation. In many instances, calcium oxalate stones have a center (nidus) composed of uric acid. Uric acid is the end product of the breakdown of purine, a product of digestion of nucleic acid in food (meat, poultry and fish) or tissue breakdown. Therefore, limiting the intake of animal protein may be a useful therapeutic approach for prevention of kidney stone formation.
Uric acid forms crystals in highly acidic urine. This is another contributing factor linking a high (animal) protein diet to increased acid load and increased uric crystal stone formation, compounding the impact of uric acid release
The Bottom Line
Many factors impact kidney stone formation, including electrolyte imbalances. Stone formation is usually not caused by a single electrolyte but rather it is likely a combination of factors impacting electrolyte balance, including nutrient depletion and dehydration. Diets low in plant-based foods and high animal protein tend to increase risk of stone formation due to an interplay of factors. Risk of kidney stone formation should be evaluated by weighing the impact of poor dietary choices combined with environmental factors, genetics, gut integrity and microbiome balance.
This is the foundation of our integrative approach to managing and prevention of kidney stones. Practitioners working with individuals to prevent kidney stone formation should formulate a personalized approach that modifies all relevant components in their comprehensive approach for best outcomes.