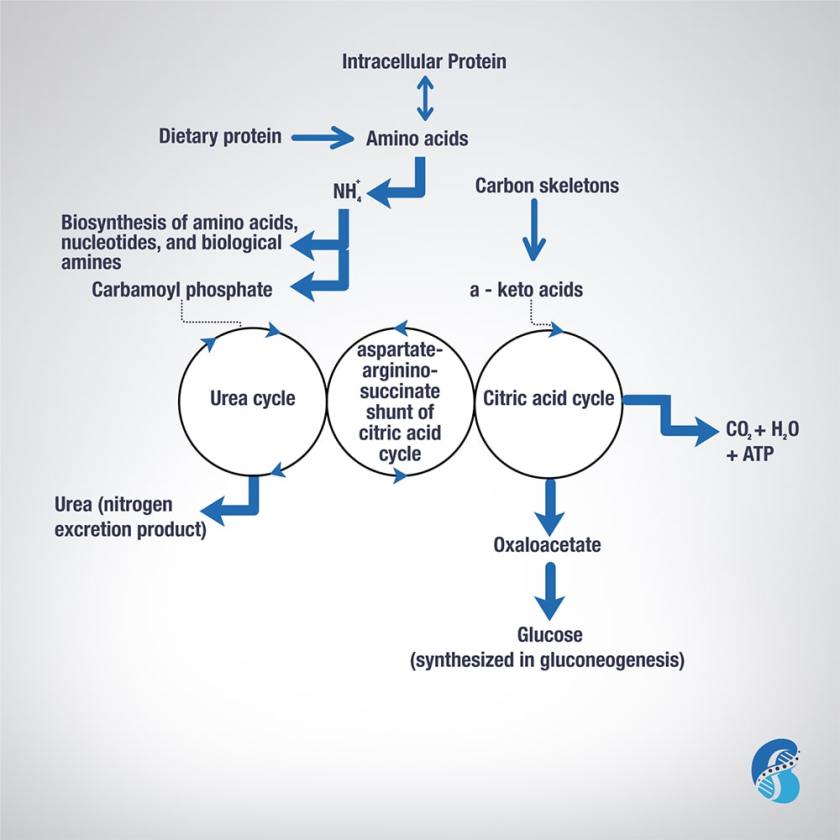
To understand the relevance of ketoacid analogues in chronic kidney disease, one need to learn the biochemistry of amino acids (AA) metabolism. This blog will attempt to describe it in short.
Excess AA in a protein-rich diet is metabolized through a process called transamination. This involves the removal of the nitrogen (or the amino group NH2 side chain) and replacing it with a keto group carbon structure forming a ketoacid or ketoacid analogue.
Apart from lysine and threonine, every EAA has a keto-analogue. The ketoacid then undergoes oxidation to carbon dioxide, water, and a three to four-carbon unit that can be converted by the liver into glucose (by gluconeogenesis) or into ketone bodies for energy.
The conversion of an amino acid to ketoacid depends on the enzyme aminotransferase. This enzyme is reversible (meaning it can convert amino acid to ketoacid or a ketoacid to amino acid). The speed and direction of this conversion depends on the amount of amino acids available, the amount of keto-acids formed, and the availability of the cofactors needed to power the process. These cofactors are nutrients that facilitate the reactions. Vitamin B6 is a major cofactor for this enzyme. Other cofactors needed in the amino acid breakdown (catabolism) include biotin, folate, and B12.
Now, remember that amino group (-NH2)? That can be used in the biosynthesis of non-essential AA and the excess ends up metabolized in the urea cycle to form urea and other nitrogen waste products that are excreted by the kidneys. It is worth noting that the AA arginine acts as a regulator of the urea cycle. Remember, arginine is essential for the production of nitric oxide, which plays an important role in blood pressure management. Issues with the urea cycle and nitric oxide balance may explain why kidney patients have blood pressure problems and may not feel well with arginine supplementation.
Overall, this process is summarized in this figure.