August Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the August Research and News.
Early Cognitive Impairment Linked to Accelerated Kidney Function Decline
In a prospective cohort of 5,761 World Trade Center responders (mean age ~54 years) without baseline CKD, this study found that cognitive impairment (CI), defined by a Montreal Cognitive Assessment (MoCA) score ≤23, was independently associated with rapid eGFR decline and new-onset CKD over a mean follow-up of 4.2 years.
Rapid kidney decline occurred in 10% of participants, and 4% developed CKD. Individuals with CI and dementia had significantly higher adjusted hazards for rapid eGFR decline (aHR: 1.63 and 2.42) and for incident CKD (aHR: 1.72 and 2.77, respectively), even after adjusting for demographics, comorbidities, WTC exposure, and mental health factors.
These associations held across multiple sensitivity analyses.
Why is this important?
This study identifies cognitive impairment, not traditionally recognized as a renal risk factor, as a strong and independent predictor of kidney function decline and CKD onset in middle-aged adults.
These findings suggest that CI may signal systemic vulnerability, perhaps via shared vascular or inflammatory pathways. Recognizing cognitive dysfunction as a contributor to kidney decline offers a potential opportunity for early risk stratification and intervention, particularly in populations with high occupational or environmental exposures like WTC responders.
Integrating cognitive screening into nephrology care may help identify patients at elevated risk earlier in the disease course.
Potassium Intake and Blood Pressure: How Much Matters for Hypertension?
This dose–response meta-analysis of 10 randomized controlled trials (2000–2024) evaluated the effect of potassium supplementation on blood pressure using 24-hour urinary potassium excretion as a biomarker.
Results showed that the impact of potassium intake on blood pressure varied by hypertension status. In normotensive individuals, a 50 mmol/day increase in potassium reduced systolic blood pressure (SBP) by only 0.5 mmHg and diastolic BP (DBP) by 0.12 mmHg.
However, in individuals with hypertension, the same increase led to a significant reduction of 5.3 mmHg in SBP and 3.62 mmHg in DBP. These findings were consistent across linear and spline regression models.
Why is this important?
With global hypertension prevalence doubling since 1990, non-pharmacologic interventions are critical. While sodium reduction is well-established, this study underscores the underappreciated potency of potassium, especially in hypertensive individuals, as a blood pressure-lowering nutrient.
The clear dose–response relationship supports updating dietary recommendations to emphasize potassium intake as a targeted intervention in hypertension management. Yet, the modest effects in normotensive people highlight the need for personalized dietary strategies.
Hidden in the Tap: Brominated Water Contaminants Raise CKD Risk Below Legal Limits
A prospective cohort study of over 89,000 women from the California Teachers Study found that long-term exposure to brominated trihalomethanes (THMs), a group of water disinfection byproducts, was significantly associated with increased risk of chronic kidney disease (CKD), even at concentrations below current U.S. regulatory limits.
Participants with brominated THM exposure at or above the 95th percentile (≥30.0 μg/L) had a 43% higher risk of CKD compared to those with low exposure (<0.7 μg/L).
Brominated THMs accounted for over half of the observed CKD risk in mixture models, far exceeding the contributions of uranium, arsenic, and chloroform.
The study also linked chronic exposure to bromodichloromethane with proximal tubular damage and reduced glomerular filtration rate based on prior toxicological evidence.
Why is this important?
These findings challenge the safety of current regulatory thresholds for water disinfection byproducts and suggest that legally "safe" levels of brominated THMs may still pose long-term nephrotoxic risk.
As the burden of CKD grows globally, especially in disproportionately impacted Black and Hispanic communities, this research underscores the urgent need for policymakers to revisit and differentiate regulations for individual THM species.
Clinicians should also consider environmental exposures like drinking water contaminants in risk assessments for kidney disease, particularly in vulnerable populations.
Join us to end the kidney disease epidemic
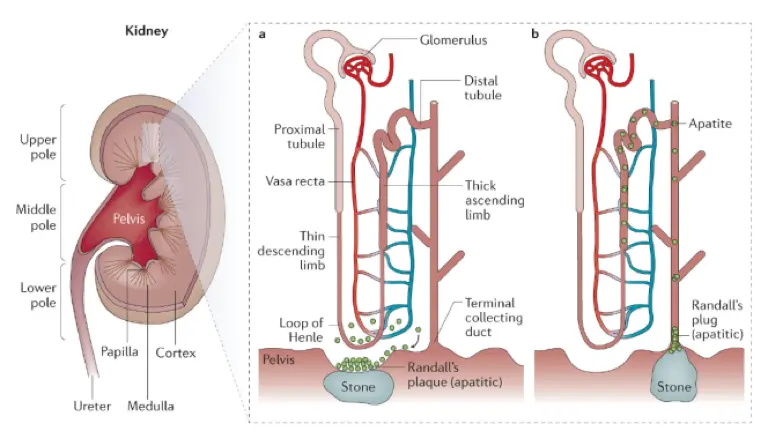
Unraveling the Protein Signature of Kidney Stones: Uromodulin Offers Protection, SSC4D Increases Risk
In the largest proteomics study of its kind, researchers evaluated over 35,000 UK Biobank participants to identify circulating proteins associated with kidney stone disease.
They found nine proteins significantly linked to stone risk, with two standing out: lower levels of uromodulin (UMOD) and higher levels of scavenger receptor cysteine-rich domain-containing group B protein (SSC4D).
Mendelian randomization confirmed these associations were likely causal, and results were replicated in the Mayo Clinic Biobank and supported by transcriptomic and GWAS data.
Notably, UMOD expression in the thick ascending limb of the nephron and its levels in blood and urine were inversely associated with stone formation, while SSC4D levels were positively associated.
Why is this important?
This study highlights how kidney stone risk may be driven by systemic protein imbalances, not just urinary solute concentration. Uromodulin is a glycoprotein produced by the kidney that plays a protective role by inhibiting crystal aggregation and modulating immune responses. Its deficiency may disrupt normal urinary defense mechanisms, increasing stone risk.
In contrast, SSC4D, an immune-related protein involved in extracellular matrix remodeling, may promote an inflammatory or fibrotic environment conducive to stone formation.
By identifying these mechanistic links, the study opens the door for biomarker-based screening and targeted therapies to prevent or slow kidney stone disease, even in individuals with preserved kidney function.
Antiemetics and the Hidden Risk: A Link to New-Onset Chronic Kidney Disease
In this large cohort study of 323,970 U.S. veterans with normal kidney function, researchers examined whether initiating antiemetics, a common class of medications used to treat nausea and vomiting, was linked to new-onset chronic kidney disease (CKD).
Among 13,154 veterans who started antiemetics, these individuals had a significantly higher risk of developing CKD over time.
This association held true across multiple statistical models, including adjusted Cox regression and propensity score methods, with hazard ratios ranging from 1.22 to 1.28.
Why is this important?
This study identifies a strong association, not necessarily a causal relationship, between antiemetic use and increased CKD risk. In other words, while antiemetic users were more likely to develop CKD, the study design cannot prove that the medications themselves directly caused kidney damage.
The increased risk may be influenced by other underlying factors, such as comorbid conditions or the reasons antiemetics were prescribed in the first place.
Still, the consistent signal across analytical approaches suggests a potential nephrotoxic effect that warrants further investigation.
Clinicians should be aware of this association and consider regular kidney function monitoring in patients on long-term antiemetics, especially those with additional CKD risk factors.
Review article of the month
Precision Nutrition in CKD
Precision nutrition in chronic kidney disease (CKD) involves tailoring dietary recommendations to an individual's genetic, biological, and environmental characteristics, moving beyond the generalized, one-size-fits-all guidelines commonly used today.
While standard guidelines suggest interventions like low-protein diets to slow CKD progression, they often fail to account for personal differences in metabolism, age, and physiology.
Precision nutrition, distinct from but related to personalized nutrition, is more data-driven and incorporates advanced tools like nutrigenetics, microbiome profiling, metabolomics, and artificial intelligence to inform individualized care. This evolving approach offers promising opportunities to improve dietary management and outcomes in CKD patients by aligning nutrition strategies with each person’s unique biology and context. It is summarized in this review article.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
Lifestyle, Stones, and Stages: What New Research Reveals About Slowing ADPKD Progression
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic condition marked by progressive cyst growth and, ultimately, kidney failure. While the underlying mutations are inherited, growing research suggests that modifiable lifestyle factors, such as diet, body weight, and metabolic health, can significantly influence ADPKD progression. A wave of recent studies is helping to define which interventions may help slow the march toward kidney failure, offering renewed hope for patients seeking preventive strategies.
This article summarizes five key studies that examine the relationship between lifestyle factors and the progression of ADPKD.
By Majd Isreb, MD, FACP, FASN, IFMCP
Obesity’s Role Depends on Disease Stage
Drawing on data from the HALT-PKD trials, researchers found that obesity was associated with a significantly increased risk of ESKD in early-stage ADPKD patients (eGFR ~91 mL/min/1.73m²), but not in late-stage patients (eGFR ~48 mL/min/1.73m²).
This stage-specific effect highlights a potential therapeutic window during the early course of the disease when weight management could have the most significant impact. Early intervention may help reduce cyst growth and slow structural kidney damage before irreversible decline sets in.
Lower BMI Linked to Slower ADPKD Progression
Another cross-sectional study of 3,556 patients with ADPKD from the US Renal Data System and the Japanese Society for Dialysis Therapy revealed that patients in Japan, who had significantly lower average BMI (22.0 vs. 28.2 kg/m²), initiated renal replacement therapy (RRT) at a later age (61.6 vs. 56.6 years, P<.001). The analysis revealed that a lower BMI was independently associated with a delayed onset of end-stage kidney disease (ESKD), even after adjusting for confounding variables and across ethnic subgroups.
This finding reinforces the importance of body weight as a modifiable factor in ADPKD. While genetics drives cyst formation, metabolic health may shape how rapidly the disease progresses. The study also suggests that national lifestyle and dietary patterns can significantly influence outcomes.
Time-Restricted Eating: A Pilot Study with Mixed Outcomes
In a 12-month randomized trial, researchers investigated whether time-restricted eating (TRE), which involves consuming all meals within an 8-hour window, could affect kidney volume or weight in patients with ADPKD. Compared to a control group that received general healthy eating guidance, both groups achieved modest weight loss. However, there was no significant difference in total kidney volume changes, as measured by MRI.
The results suggest that the benefits of TRE may stem more from general weight reduction than from meal timing itself. Although this trial was limited in size and duration, it contributes to the growing evidence that lifestyle modification, particularly involving caloric intake and weight control, can help manage ADPKD progression.
Join us to end the kidney disease epidemic
Silent Stones Accelerate Decline in Kidney Function and ADPKD Progression
A retrospective cohort study involving 195 patients with ADPKD found that those with asymptomatic kidney stones experienced a significantly faster decline in estimated glomerular filtration rate (eGFR) compared to those without nephrolithiasis. The presence of kidney stones independently predicted poorer outcomes.
This finding challenges the assumption that asymptomatic stones are clinically insignificant. Proactive monitoring and management of nephrolithiasis, including the use of urine alkalinizing agents like potassium citrate or KetoCitra, may help slow the decline in kidney function and improve long-term outcomes.
Alkali-Rich Diets May Protect the Kidney in Early CKD
In a randomized controlled trial of 153 patients with stage G1 CKD and normal eGFR, dietary interventions that reduced acid load, either through increased consumption of fruits and vegetables or sodium bicarbonate, led to reductions in biomarkers of kidney injury over five years.
Although this study was not explicitly focused on ADPKD progression, its findings align with the broader view that dietary acid load has an impact on kidney health. An alkali-rich diet may be an easily accessible, low-risk strategy to help preserve kidney function in early stages of disease, including in genetically driven conditions like ADPKD.
The Bottom Line on Integrating Lifestyle for Slowing ADPKD Progression
Together, these studies reinforce a critical message: the progression of ADPKD is not solely determined by genes. Lifestyle factors, particularly body weight, dietary composition, and vigilance regarding silent complications, play a significant role in shaping outcomes.
While more long-term research is needed, the evidence already suggests an emerging paradigm in ADPKD care: one that combines genetic awareness with proactive, personalized lifestyle interventions. Patients and clinicians alike can take heart in knowing that the trajectory of this condition can, in part, be altered by informed, daily choices.
The Brain-Gut-Kidney Axis: A New Frontier in Hypertension and Chronic Kidney Disease
Chronic kidney disease (CKD) and hypertension are two closely linked conditions that affect millions worldwide. Traditionally, they have been viewed as issues related to blood pressure regulation and kidney function. But emerging research is pointing to a deeper and more complex connection, one that links the brain, gut, microbiota, and kidneys. This interconnected system, known as the brain-gut-kidney axis, could open new doors for understanding, preventing, and treating CKD and hypertension.
The Microbiota Connection
Our gut is home to trillions of microbes that play crucial roles in digestion, immunity, and metabolism. When this microbial ecosystem is disrupted, a condition known as gut dysbiosis can trigger a chain reaction throughout the body. In CKD, gut dysbiosis has been linked to inflammation, metabolic changes, and immune dysfunction, all of which can worsen kidney health and elevate blood pressure.
What Is the Brain-Gut-Kidney Axis?
The brain-gut-kidney axis refers to a bi-directional communication network that connects the central nervous system, the gastrointestinal tract, and the kidneys. Here’s how the system works:
-
The brain regulates the autonomic nervous system and stress response, influencing gut motility and permeability.
-
The gut microbiota produces metabolites that can influence brain and kidney function, both directly and through immune and metabolic signaling.
-
The kidneys, in turn, affect gut health through uremia-induced changes in the intestinal barrier and microbial composition.
When any part of this axis becomes dysregulated due to factors such as poor diet, stress, medication, or disease, it can have ripple effects throughout the entire system.
Why This Matters in CKD and Hypertension
Both CKD and hypertension are associated with systemic inflammation, sympathetic nervous system overactivity, and metabolic disturbances. These are all areas where the brain-gut-kidney axis plays a central role. For example:
-
Immune dysregulation in CKD may be fueled by microbial products crossing a leaky gut barrier.
-
Sympathetic activation,a hallmark of hypertension, may be triggered or worsened by inflammatory signals from the gut.
-
Microbial metabolites such as short-chain fatty acids (SCFAs) or uremic toxins (e.g., indoxyl sulfate, p-cresol) can either protect or harm the kidneys, depending on the microbial balance.
-
Join us to end the kidney disease epidemic
A New Therapeutic Horizon
Understanding the brain-gut-kidney axis opens the door to novel treatments that go beyond blood pressure medications and dialysis. Future therapies may include:
-
Targeted probiotics and prebiotics to restore a healthy microbiome
-
Gut barrier protectants to reduce systemic inflammation
-
Neuromodulation techniques to reset autonomic balance
-
Dietary interventions designed to support microbial diversity and function
The Bottom Line on the Brain-Gut-Kidney Axis
The brain-gut-kidney axis represents a paradigm shift in how we think about hypertension and chronic kidney disease. Rather than treating these conditions in isolation, we must consider the body as an interconnected system, with the microbiota playing a central role. Ongoing research in this area is poised to revolutionize not just nephrology, but how we approach chronic disease as a whole. For a more in-depth review, read the original article here.
July Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the July Research and News.
DASH4D Trial: Sodium Reduction Plus DASH-Style Diet Lowers Blood Pressure in Type 2 Diabetes
In the DASH4D randomized crossover trial of 102 adults with type 2 diabetes, researchers tested the effects of a modified DASH-style diet optimized for diabetes (DASH4D) and sodium reduction on blood pressure.
Participants followed four controlled 5-week diets: DASH4D or a typical U.S. diet, each with high or low sodium.
Compared to a high-sodium typical diet, the DASH4D diet with reduced sodium lowered systolic blood pressure by 4.6 mm Hg and diastolic by 2.3 mm Hg. Most reductions occurred in the first 3 weeks and were mainly attributed to sodium reduction, even among those on multiple antihypertensive medications.
Why is this important?
People with type 2 diabetes are at high cardiovascular risk, and this trial demonstrates that dietary sodium reduction, even without weight loss, can meaningfully lower blood pressure. The findings reinforce the importance of dietary strategies alongside pharmacologic treatment in managing hypertension in diabetes.
Elevated TSH Causally Linked to Reduced Kidney Function: A Mendelian Randomization Study
This multivariable Mendelian randomization (MVMR) study explored the causal relationship between thyroid function and kidney health using data from over 17,000 participants in the Taiwan Biobank and was validated in European populations.
Elevated thyroid-stimulating hormone (TSH) levels and hypothyroidism were associated with lower estimated glomerular filtration rate (eGFR), while thyroid peroxidase antibodies (TPOAb) showed a positive correlation with eGFR.
MVMR confirmed a causal effect of higher TSH, but not fT4 or TPOAb, on reduced kidney function, reinforcing the role of thyroid dysfunction in chronic kidney disease (CKD).
Why is this important?
These findings suggest that thyroid dysfunction, particularly elevated TSH levels, may directly impair kidney function. Recognizing and managing thyroid abnormalities could offer a new avenue for slowing CKD progression and optimizing patient care.
Reducing Dietary Phosphorus Additives Lowers FGF23 and PTH in Adults with CKD
This controlled feeding study examined how cutting phosphorus additives in the diet affects mineral metabolism in 50 adults with and without CKD.
After two weeks on a phosphorus–additive–enhanced diet, participants transitioned to a low-additive diet for six weeks.
In both groups, 24-hour urine phosphorus excretion dropped by ~30%. In the CKD group, fibroblast growth factor 23 (FGF23) levels declined by 25% and parathyroid hormone (PTH) by 20%.
Among healthy individuals, FGF23 decreased significantly only in White participants, not in Black participants, indicating a race-based difference in response.
Why is this important?
Excess dietary phosphorus from additives may worsen mineral imbalances that contribute to CKD progression and cardiovascular risk. This study shows that reducing phosphorus additives can improve key metabolic markers, especially in those with CKD, highlighting a practical, diet-based strategy for managing kidney health.
Join us to end the kidney disease epidemic
Urine Alkalization Enhances Gout Management Beyond Uric Acid Control
This prospective cohort study investigated whether adding urine alkalization to urate-lowering therapy (ULT) improves outcomes in men with gout and low urinary pH (<6.2).
A total of 385 participants initiating febuxostat therapy were divided into groups based on whether they also received a citrate mixture for urine alkalization.
At 12 weeks, the alkalization group showed a significantly lower urine albumin-to-creatinine ratio (UACR), fewer gout flares, lower pain scores, and improved lipid profiles (lower triglycerides, higher HDL-C), despite requiring lower febuxostat doses. Serum urate targets and eGFR were similar between groups.
Why is this important?
While xanthine oxidase inhibitors like febuxostat lower serum urate, they don’t address urine acidity, which contributes to urate crystal formation and renal microvascular damage.
This study provides evidence that targeting urinary pH may offer complementary benefits, including reduction in albuminuria, an early marker of kidney damage, and mitigation of gout flares and metabolic dysfunction.
By reducing the need for higher drug doses and improving lipid metabolism, urine alkalization with products like Ketocitra or potassium citrate offers a low-risk, cost-effective adjunct that could help personalize gout and kidney disease management, particularly in patients with features of metabolic syndrome.
COVID-19 Vaccination and De Novo Glomerular Disease: International Registry Insights
This international registry study (IRocGN2) analyzed 98 cases of new-onset glomerular disease (GD) suspected to be temporally associated with COVID-19 vaccination, collected from 44 centers globally.
IgA nephropathy (IgAN) and minimal change disease (MCD) were the most commonly reported post-vaccine glomerular pathologies, especially following mRNA-based vaccines.
Other reported conditions included membranous nephropathy, pauci-immune GN, and collapsing glomerulopathy, sometimes presenting as dual pathologies.
Median follow-up was 89 days post-diagnosis. Patients with IgAN and MCD experienced more favorable outcomes, exhibiting higher rates of kidney function recovery and proteinuria remission at 4–6 months compared to those with other forms of GN.
Why is this important?
This study provides the most comprehensive global data to date on glomerular diseases potentially linked to COVID-19 vaccination. While causality remains unproven, the consistent temporal pattern and histologic trends, particularly the predominance of IgAN and MCD, raise important questions about immune activation pathways triggered by vaccination.
Importantly, the overall kidney outcomes were favorable, especially for these two subtypes, supporting the continued safety and utility of COVID-19 vaccination. These findings help clinicians weigh rare risks against substantial public health benefits and underscore the value of post-vaccine surveillance for vulnerable patients, including those with prior renal or autoimmune conditions.
Review article of the month
Effects of microplastics and nanoplastics on the kidney and cardiovascular system
Microplastics and nanoplastics are pervasive environmental pollutants found in air, food, and water, leading to widespread human exposure and accumulation in organs such as the heart, kidney, liver, and brain. Research in animal models and human cells shows that these particles can trigger oxidative stress, inflammation, metabolic disruption, and immune dysfunction.
This review article examines the toxic effects of these compounds on kidney and cardiovascular cells, with a heightened risk for individuals undergoing dialysis. Additionally, particulate plastic exposure has been linked to cardiovascular disease. Mitigation strategies include improved waste management, use of alternative materials, filtration technologies, and regulatory reforms, underscoring the urgent need for further research on their health impacts.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
June Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the June Research and News.
Long-Term Uranium Exposure in Drinking Water Linked to CKD Risk in Women
In a large prospective study of over 88,000 women from the California Teachers Study, researchers evaluated the effects of long-term exposure to uranium and arsenic in community drinking water on chronic kidney disease (CKD) risk.
Between 1995 and 2005, participants’ residential addresses were linked to time-weighted averages of uranium and arsenic concentrations in local water systems. From 2005 to 2018, 6,185 moderate-to-end-stage CKD cases were identified.
Uranium exposure, even at levels well below the current regulatory limit of 30 µg/L, was associated with a significantly increased CKD risk. For example, exposure levels between 10–15 µg/L carried a 33% greater risk of CKD compared to <2 µg/L. Arsenic was not significantly associated with CKD overall but showed risk in younger individuals and those with diabetes or cardiovascular disease. These findings remained robust even after adjusting for demographic, lifestyle, and socioeconomic factors.
Why is this important?
This study underscores the potential nephrotoxic effects of uranium in drinking water, even at concentrations currently deemed "safe" by regulatory standards. It calls into question whether existing guidelines are adequate to protect kidney health, especially in vulnerable populations.
As chronic kidney disease continues to rise globally, often without a clear cause, understanding and mitigating environmental exposures like low-level uranium becomes crucial. These findings advocate for stricter drinking water standards, using high-quality water filtration systems, and further investigation into chronic low-dose exposures, particularly in communities already burdened by other health disparities.
Lower BMI Linked to Slower Progression to Kidney Failure in ADPKD: Insights from the US and Japan
This cross-sectional analysis examined the relationship between body mass index (BMI) and age at initiation of renal replacement therapy (RRT) in 3,556 patients with autosomal dominant polycystic kidney disease (ADPKD) from two national registries, the US Renal Data System (n=2,491) and the Japanese Society for Dialysis Therapy (n=1,065).
Patients in Japan initiated RRT at an older average age (61.6 vs. 56.6 years, P<.001) and had significantly lower BMI (22.0 vs. 28.2 kg/m², P<.001). Across both populations, lower BMI was independently associated with the delayed onset of end-stage kidney disease requiring RRT.
This inverse relationship held true after adjusting for confounding variables and within separate ethnic subgroups. Notably, Japanese participants were the leanest and oldest at RRT initiation, suggesting a slower disease trajectory.
Why is this important?
This study underscores the critical role of metabolic health in the progression of ADPKD. While genetic factors drive cyst formation, modifiable lifestyle factors like body weight, diet, and water intake may significantly impact disease progression. The finding that lower BMI correlates with later RRT initiation suggests that weight management could be a therapeutic target to delay kidney failure in ADPKD. Differences between US and Japanese cohorts also highlight the influence of national dietary patterns and lifestyle on kidney outcomes, reinforcing the value of cross-cultural research in chronic disease prevention.
Beyond Total Vitamin D: Free and Bioavailable 25(OH)D Decline with CKD Progression and After Kidney Transplant
This study evaluated vitamin D status in 38 patients with stage 3–4 chronic kidney disease (CKD) and 38 patients with end-stage renal disease (ESRD) who were followed for six months post-kidney transplant (KT).
Researchers measured total 25-hydroxyvitamin D [25(OH)D] and vitamin D binding protein (VDBP) and calculated free and bioavailable 25(OH)D using the Bikle formula.
ESRD patients had significantly lower total, free, and bioavailable 25(OH)D levels compared to CKD stage 3–4 patients, while VDBP levels did not differ.
Six months post-transplant, free and bioavailable 25(OH)D continued to decline despite stable total 25(OH)D levels and increased VDBP. Regression analysis showed that bioavailable 25(OH)D and serum creatinine were independent predictors of CKD stage.
Why is this important?
This study highlights that total 25(OH)D levels may not fully capture vitamin D deficiency in CKD or post-transplant patients. As kidney function declines, both free and bioavailable forms of vitamin D, which are more physiologically relevant, decrease, and they continue to decline even after transplantation, possibly due to rising VDBP levels.
These findings emphasize the need for a more nuanced approach to vitamin D assessment and supplementation in CKD, shifting the focus from total to bioavailable and free 25(OH)D to better guide clinical care and potentially improve long-term outcomes.
Join us to end the kidney disease epidemic
Gut Microbiome Changes May Drive Disease Progression and Early Hypertension in ADPKD
This cross-sectional pilot study examined the gut microbiome of 25 patients with autosomal dominant polycystic kidney disease (ADPKD) and 12 matched healthy controls.
Using 16S rRNA sequencing and serum toxin analysis, the study found that patients with ADPKD had a significantly altered gut microbiota, including reduced levels of beneficial Actinobacteria (such as Bifidobacteriaceae) and elevated levels of Enterobacteriaceae.
More severe ADPKD (Mayo Class 1D/1E) was linked to increased Streptococcaceae, while early-onset hypertension (<35 years) was associated with higher Proteobacteria and lower Tannerellaceae. Serum uremic toxin levels were elevated and correlated with worsening kidney function.
Why is this important?
This study highlights a potential connection between gut dysbiosis and ADPKD progression, independent of kidney function. Specific microbiome signatures appear to align with more aggressive disease phenotypes and earlier vascular involvement.
These findings suggest that the gut microbiota may play a role in modifying disease severity in ADPKD and could serve as a novel therapeutic target for slowing progression and improving patient outcomes.
Sex Hormones Directly Influence Kidney Function: Insights from Hormone Therapy
In this prospective study, 44 individuals initiating sex hormone therapy were assessed over three months to explore how estradiol and testosterone affect kidney function.
Those undergoing feminizing therapy (estradiol plus antiandrogens) experienced increases in measured GFR and kidney perfusion, along with reductions in key tubular injury biomarkers (e.g., NGAL, EGF, MCP-1, YKL-40).
In contrast, masculinizing therapy (testosterone) was associated with increases in injury-related markers like urine YKL-40 and plasma TNFR-1.
Proteomic analysis revealed differential regulation of hundreds of proteins, many of which were kidney-protective and positively associated with estradiol but suppressed by testosterone.
Why is this important?
This study provides mechanistic evidence that sex hormones significantly influence kidney physiology, supporting the clinical observation that women often experience slower CKD progression than men.
Estradiol appears to enhance renal perfusion and reduce injury, while testosterone may promote damage. These findings help explain sex differences in kidney disease progression and suggest that hormone modulation could inform precision nephrology strategies tailored by sex or gender identity.
Review article of the month
Optimizing Protein Intake in CKD: Balancing Quantity, Quality, and Long-Term Adherence
This review emphasizes the importance of dietary protein management in chronic kidney disease (CKD), highlighting both the quantity and quality of protein intake. Since the 1930s, protein restriction has been used to manage uremic symptoms, and modern research confirms that excessive protein, especially from animal sources, can worsen kidney damage by increasing uremic toxin production.
In contrast, plant-based diets, when properly balanced to provide all essential amino acids, can reduce toxin burden and offer health benefits. The article discusses strategies to optimize protein intake and ensure long-term adherence to dietary recommendations to improve CKD outcomes.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
May Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the May Research and News.
Magnesium and Chronic Kidney Disease: An Essential Consideration
An expert article by our friend Lindsey Zirker, MS, RD in Renal & Urology News highlights magnesium’s underappreciated role in CKD management. Beyond regulating calcium and potassium, magnesium modulates inflammation, insulin sensitivity, and vascular tone. Yet serum levels may not tell the whole story—clinicians are encouraged to evaluate magnesium status more comprehensively, including dietary intake, medications (e.g., PPIs, diuretics), and intracellular levels (e.g., RBC magnesium).
Why is this important?
Maintaining magnesium within the optimal 1.7–2.3 mg/dL range could help mitigate complications in CKD. The choice of supplement matters—magnesium citrate, glycinate, or malate are preferred forms with better absorption and fewer GI side effects.
Proinflammatory Diets Linked to Higher CKD Risk
A review in the Journal of Health, Population and Nutrition analyzed 13 cross-sectional studies and found that individuals with higher dietary inflammatory index scores had significantly greater odds of CKD (OR 1.36) and reduced eGFR (OR 1.58).
Why is this important?
Dietary inflammation is modifiable. This evidence strengthens the case for anti-inflammatory dietary patterns—rich in fruits, vegetables, legumes, and omega-3s—in both prevention and management of CKD. Personalized nutrition approaches that assess dietary inflammatory load could help reduce progression risk.
Weight Loss Without Drugs or Surgery in Advanced CKD: What Works?
A new scoping review in Kidney Medicine reviewed 17 studies involving patients with stage 4–5 CKD and obesity. Non-pharmacological, non-surgical interventions—particularly caloric restriction diets supported by lifestyle coaching—produced meaningful weight loss (up to 7 kg or 15.4 lbs) with no reported adverse events.
Why is this important?
Obesity in CKD complicates transplant candidacy, increases cardiovascular risk, and worsens inflammation. This review supports a role for structured lifestyle programs in advanced CKD populations, challenging the belief that significant weight loss is unsafe or unachievable without surgery.
Join us to end the kidney disease epidemic
Drug-Induced Membranous Nephropathy: Mechanisms and Genetics
An in-depth review in JASN explores the pathophysiology of drug-induced membranous nephropathy, triggered by agents like gold salts, NSAIDs, mercury, D-penicillamine, lipoic acid, and thiol-containing drugs. The review notes variable T-cell responses and genetic susceptibility in animal models.
Why is this important?
Understanding the immune-genetic interface in drug-induced nephropathy may lead to personalized risk assessment and earlier intervention. Integrative providers should review all supplements and medications in patients with unexplained proteinuria.
Ultra-Processed Foods and Premature Death: A Call for Reform
A large epidemiologic study in the American Journal of Preventive Medicine (reported by CNN) found that each 10% increase in calories from ultra-processed foods was associated with a 3% higher risk of premature death. The U.S. leads global consumption, with nearly 55% of calories coming from these foods.
Why is this important?
This study reinforces how dietary quality, not just nutrients, but also food processing, directly impacts longevity. For CKD patients, reducing ultra-processed intake could reduce mortality, cardiovascular events, and mental health disorders.
Review article of the month
Gut-Liver-Kidney Axis and Metabolic Disease: Microbiome at the Center
A CJASN perspective piece explores how gut dysbiosis, small intestine bacterial overgrowth, and gut-derived metabolites link MASLD (formerly NAFLD) and CKD. Emerging omics-based research reveals overlapping inflammatory and metabolic pathways.
The gut-kidney axis remains a promising target for intervention. A comprehensive gut restoration protocol and microbiome-targeted therapies may offer multi-organ benefits in patients with metabolic dysfunction. This piece adds weight to the integrative focus on gut health in chronic disease.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
April Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the April Research and News.
Can Time-Restricted Eating Slow Kidney Disease Progression in ADPKD Patients?
In a pioneering study published in the Clinical Kidney Journal by Cortney Steele et al., researchers explored the impact of time-restricted eating (TRE) on autosomal dominant polycystic kidney disease (ADPKD).
This 12-month randomized trial compared the effects of TRE, where participants ate within an 8-hour window daily, against a control group receiving general healthy eating advice without time restrictions. The study assessed adherence rates, changes in total kidney volume measured by MRI, and weight changes among 29 participants.
Results indicated modest weight loss in both groups, with no significant differences in kidney volume change between TRE and control groups, suggesting that weight and adiposity reductions might be more influential in managing kidney disease progression than the specific timing of meals.
Why this is important:
This study highlights the potential and limitations of dietary timing as a therapeutic strategy in managing ADPKD. While TRE did not achieve significant improvements over general healthy eating advice in terms of kidney volume, the observed weight loss associated with both diets suggests that broader dietary and lifestyle modifications might play a crucial role in slowing disease progression.
These findings open avenues for further research to explore how different dietary strategies can be optimized for better health outcomes in ADPKD patients, emphasizing the importance of personalized dietary interventions.
One major critique of this study is its short-term duration while ADPKD has progressed over the decades.
Revitalizing Renal Health: High-Intensity Interval Training as a Preventive Strategy Against CKD in Older Adults
A groundbreaking study conducted by Hallan et al. explores the under-researched area of physical exercise's impact on kidney function, particularly in older adults.
This randomized clinical trial, derived from the Generation 100 Study in Trondheim, Norway, assessed the efficacy of different exercise intensities in preserving kidney function over five years.
Participants, aged 70-77, engaged in either moderate-intensity continuous training, high-intensity interval training (HIIT), or followed standard physical activity guidelines.
Results strikingly indicated that HIIT significantly reduced the risk of rapid kidney function decline compared to the control group, showcasing a potential preventive measure for chronic kidney disease (CKD) akin to its benefits in cardiovascular health.
Why this is important:
As CKD emerges as a significant global health concern, particularly among aging populations, understanding and mitigating its risk factors is crucial. This study highlights physical exercise, specifically high-intensity interval training, as a potent, non-pharmacological intervention that can substantially reduce the progression of CKD.
These findings suggest that integrating targeted exercise regimens could enhance longevity and quality of life by preserving kidney function, potentially shifting public health approaches toward more proactive, lifestyle-oriented strategies in managing CKD risk.
Silent Stones: The Impact of Asymptomatic Kidney Stones on Disease Progression in ADPKD
In a study published in BMC Nephrology, researchers investigated the influence of asymptomatic kidney stones on disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD).
This retrospective cohort study followed 195 ADPKD patients, distinguishing between those with nephrolithiasis (85 patients) and those without (110 patients). The study aimed to understand the relationship between nephrolithiasis and the rate of kidney function decline, measured by changes in the estimated glomerular filtration rate (ΔeGFR).
Results indicated a significantly greater decline in kidney function among patients with nephrolithiasis compared to those without. Furthermore, nephrolithiasis was found to be an independent predictor of faster kidney function decline, emphasizing the need for vigilant monitoring of kidney stones in ADPKD patients to potentially slow disease progression and preserve renal function.
Why this is important:
The findings underscore the clinical significance of asymptomatic nephrolithiasis in ADPKD, a condition previously considered only a minor complication relative to cyst development.
By demonstrating that nephrolithiasis contributes independently to faster kidney function decline, this study prompts a reevaluation of monitoring and management strategies for ADPKD patients.
It suggests that proactive detection and treatment of kidney stones, even when asymptomatic, could be crucial in delaying the progression to end-stage kidney disease, thus improving outcomes and quality of life for patients afflicted with this genetic disorder.
Furthermore, the study indicates that alkalinizing the urine with products such as potassium citrate or KetoCitra may have another role in slowing the progression of ADPKD.
Join us to end the kidney disease epidemic
Broadening Horizons: Effects of a High-Diversity Plant-Based Diet on CKD Patients' Health and Gut Microbiome
In a pioneering study published in the Clinical Journal of the American Society of Nephrology, investigators explored the effects of a diverse plant-based diet on adults with stage 3-4 chronic kidney disease (CKD).
This randomized controlled trial compared the impacts of a high-diversity plant-based diet (HDPD, featuring over 30 unique plant foods weekly) and a low-diversity plant-based diet (LDPD, with fewer than 15 unique plant foods weekly), each followed for six weeks with a washout period in between.
Key findings included improvements in diet quality and significant reductions in uremic toxins among HDPD responders, particularly those with advanced CKD.
The HDPD also enhanced fecal microbiome diversity, increasing beneficial metabolites like butyrate, and decreased overall symptom burden, including constipation.
In contrast, the LDPD led to reduced microbial diversity and abundance. These results underscore the potential of a varied plant-based diet to ameliorate CKD symptoms and alter gut microbiota composition favorably.
Why this is important:
This study highlights the profound influence of dietary diversity on health outcomes in CKD patients, challenging traditional dietary restrictions often prescribed in CKD management.
By demonstrating that a varied plant-based diet not only manages but potentially improves various clinical and microbiological parameters, this research advocates for a paradigm shift towards more inclusive dietary guidelines in CKD care.
Such dietary strategies may provide a non-pharmacological approach to managing CKD progression and symptomatology, emphasizing the role of diet as a cornerstone of integrated disease management. This could significantly enhance patient quality of life and offer a customizable, accessible intervention to mitigate CKD progression.
Review article of the month
Efficacy of Risk Factor-Based Screening for Chronic Kidney Disease in Primary Care
This systematic open-access review evaluates the effectiveness of risk factor-based screening for early detection of chronic kidney disease (CKD) in adults in primary care settings, synthesizing evidence from 24 studies across 11 countries.
Utilizing varied screening methods like estimated glomerular filtration rate (eGFR), albumin-creatinine ratio (ACR), and dipstick urinalysis, the review highlights that such screenings can identify a significant percentage of individuals with previously undiagnosed CKD, with prevalence rates of reduced kidney function and confirmed CKD ranging from 2.9% to 56% and 4.4% to 17.1%, respectively.
The findings suggest increased patient referrals and satisfaction, although the review notes limitations such as the lack of a meta-analysis and limited generalizability to resource-poor settings. The authors call for future studies with robust designs and comprehensive interventions to enhance the effectiveness of CKD screening in primary care.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
March Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the March Research and News.
Muscle Strength Deterioration: A Critical Factor in Chronic Kidney Disease Progression
A study by Antoine Chatrenet and colleagues, published in the Clinical Kidney Journal, explores the impact of chronic kidney disease (CKD) on the relationship between skeletal muscle mass and muscle strength, highlighting an often-overlooked aspect of CKD progression.
The study analyzed data from 1098 patients, assessing appendicular skeletal muscle mass (ASM) and maximal voluntary force (MVF) across different stages of CKD.
Findings reveal that muscle force declines in later stages of CKD (G3 to G5) independently of muscle mass, indicating a deterioration in muscle quality rather than quantity.
Despite similar rates of sarcopenia across all CKD stages, patients in the early stages (G1-G2) exhibited a higher likelihood of recovery.
Why is this important?
This research underscores the importance of evaluating muscle function, not just mass, in patients with CKD. The decline in muscle strength, irrespective of muscle mass, suggests that interventions to improve muscle quality could be crucial.
Understanding these dynamics can lead to more targeted therapies and rehabilitation programs aimed at preserving muscle function, thereby enhancing the quality of life and potentially slowing CKD progression.
This study emphasizes the need for early intervention in CKD to mitigate neuromuscular impairments that contribute to morbidity.
Glomerular Hyperfiltration as a Risk Factor for Cardiovascular Disease in Type 2 Diabetes
A study led by Seung Min Chung, published in the Clinical Journal of the American Society of Nephrology, evaluates the impact of glomerular hyperfiltration (GHF) on cardiovascular disease (CVD) risk among patients with type 2 diabetes mellitus.
The retrospective cohort study analyzed 1,952,053 patients, classifying them based on their estimated glomerular filtration rate (eGFR) into different percentile groups to observe the incidence of CVD, which included myocardial infarction, stroke, and heart failure.
Results indicated an inverted J-shaped relationship between eGFR and CVD, with the highest risks observed in the lowest and highest eGFR percentiles, highlighting GHF as a significant risk factor, especially for myocardial infarction and heart failure.
Why is this important?
Glomerular hyperfiltration (GHF) is one of the earliest changes in diabetic kidney disease. This study demonstrated that GHF is a significant risk factor for cardiovascular disease.
Typically overlooked in clinical assessments, GHF is linked with an elevated risk of severe cardiovascular events, suggesting a need for early detection and targeted interventions to mitigate these risks.
Understanding and addressing GHF can lead to improved cardiovascular outcomes and provide critical insights into the progression of kidney disease in diabetic patients, emphasizing the necessity for a shift in clinical focus towards the early stages of renal changes.
Dietary Inflammation and Chronic Kidney Disease Risk: Insights from the Comprehensive Dietary Inflammation Index
A recent published in the Clinical Journal of the American Society of Nephrology introduces a novel dietary inflammatory score— the Comprehensive Dietary Inflammation Index (CDII)—to assess the link between diet and the onset of chronic kidney disease (CKD).
Developed using data from 9,814 participants initially free of CKD in the Atherosclerosis Risk in Communities Study, the CDII quantifies dietary inflammation based on the consumption of eight specific food groups.
Over 19 years, participants adhering to a pro-inflammatory diet, as indicated by higher CDII scores, exhibited a 28% increased risk of developing CKD, suggesting that dietary inflammation plays a significant role in CKD onset.
Why is this important?
The findings underscore the importance of dietary choices in managing inflammation and preventing chronic kidney disease.
By identifying pro-inflammatory diets as a modifiable risk factor, this study provides a practical tool—the CDII—for clinicians and patients to assess and adjust dietary habits.
Reducing the intake of pro-inflammatory foods could emerge as a key strategy to mitigate CKD risk, offering a preventive approach that can be integrated into broader dietary recommendations and public health policies.
Join us to end the kidney disease epidemic
Impact of Body Mass on Kidney Disease Progression in Autosomal Dominant Polycystic Kidney Disease
A study by Kristen L. Nowak et al., published in the Clinical Journal of the American Society of Nephrology, investigates the effect of body mass index (BMI) on the progression to end-stage kidney disease (ESKD) in patients with autosomal dominant polycystic kidney disease (ADPKD).
Utilizing data from the HALT PKD trials, the study divides participants into early-stage (eGFR: 91±17 mL/min/1.73m2) and late-stage (eGFR: 48±12 mL/min/1.73m2) groups. It was found that obesity significantly increased the risk of ESKD in early-stage ADPKD but not in late-stage patients.
This differentiation highlights the nuanced role of body weight in the progression of ADPKD, particularly emphasizing the importance of managing obesity early in the disease trajectory to mitigate risks.
Why is this important?
The findings highlight a crucial window during the early stages of ADPKD, during which weight management could play a pivotal role in slowing the progression to ESKD.
Understanding how BMI influences disease outcomes could lead to more targeted interventions and inform clinical guidelines, potentially improving long-term outcomes for patients with ADPKD. This study underscores the importance of personalized treatment approaches based on disease staging and individual risk factors such as obesity.
Review article of the month
Music Therapy Slows Cognitive Decline in CKD
This scoping review published in Nephrology Dialysis Transplantation investigates the clinical application and benefits of music-based interventions (MBIs) in patients with mild cognitive impairment (MCI) and explores their potential use in chronic kidney disease (CKD) populations.
The systematic search included sixteen studies, predominantly randomized control trials, assessing the effects of various MBIs, ranging from passive music listening to active music-making.
The findings suggest significant cognitive improvements and behavioral benefits from these interventions in MCI patients, with active interventions showing greater cognitive benefits and receptive approaches aiding behavioral symptoms.
Despite the absence of studies focusing specifically on CKD patients, the high prevalence of MCI within this group indicates a critical area for future research on the application of MBIs to potentially improve cognitive and depressive symptoms in CKD patients.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
February Research and News 2025
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the February Research and News.
Alkali-Rich Diets in CKD: A Shield Against Kidney Injury
A pivotal study by Nimrit Goraya et al., published in Kidney International Reports, investigated the effects of dietary acid reduction in patients with stage G1 chronic kidney disease (CKD) who maintain a normal estimated glomerular filtration rate (eGFR).
This randomized trial involved 153 participants who received interventions aimed at reducing dietary acid through increased consumption of fruits and vegetables or oral sodium bicarbonate, compared to usual care, over five years.
Results demonstrated that these interventions significantly reduced acid accumulation and lowered biomarkers of kidney injury, suggesting that dietary modifications could play a crucial role in protecting kidney function in early-stage CKD patients.
Why is this important?
This research underscores the potential of dietary interventions in managing CKD at an early stage, particularly among patients with a normal eGFR.
By reducing dietary acid load, the study suggests a proactive approach to delay or prevent the progression of kidney damage, offering a non-invasive strategy that could be easily implemented in daily life.
The findings highlight the importance of dietary management in CKD, paving the way for guidelines that could include dietary modifications as a standard recommendation for early-stage CKD patients to maintain kidney health and function.
Elevated Urinary Arsenic and Its Impact on Kidney Health in US Adults
Investigators in this research published in the Journal of Trace Elements in Medicine and Biology, December 2024, looked at the association between urinary arsenic levels and kidney damage among US adults from 2007 to 2018 using data from NHANES.
Employing multivariable logistic regression models, the study reveals that higher quartiles of urinary arsenic significantly correlate with increased risks of kidney damage, particularly albuminuria and hyperuricemia.
The research underscores a concerning link between elevated arsenic levels, primarily due to environmental exposure, and various indicators of kidney damage, suggesting an urgent need for further investigative and preventive measures.
Why is this Important?
This study is critical as it highlights the potential public health implications of arsenic exposure on kidney health, an issue that may be underrecognized in environmental health policies.
Understanding the relationship between arsenic exposure and kidney damage not only helps in early diagnosis and treatment but also underscores the importance of regulating and monitoring environmental contaminants to prevent chronic kidney diseases.
This necessitates more stringent environmental protections and targeted public health initiatives to reduce arsenic exposure in vulnerable populations.
Nicotinamide Riboside and Coenzyme Q10: Boosting Mitochondrial Health in Chronic Kidney Disease
A study by Ahmadi et al., published in the Clinical Journal of the American Society of Nephrology on January 23, 2025, explored the impact of Nicotinamide Riboside (NR) and Coenzyme Q10 (CoQ10) on inflammation, oxidative stress, and mitochondrial function in individuals with chronic kidney disease (CKD).
Conducted as a pilot randomized, double-blind, placebo-controlled crossover trial with 25 participants suffering from moderate-to-severe CKD, the research demonstrates that a 6-week supplementation of 1200 mg/day of CoQ10 and 1000 mg/day of NR significantly improves oxidative stress markers and inflammatory profiles.
The study further elucidates that NR enhances mitochondrial bioenergetics in immune cells, suggesting potential therapeutic benefits in managing CKD-related complications.
Why is this important?
The findings of this study are pivotal as they provide preliminary evidence that targeting mitochondrial dysfunction through specific supplements like NR and CoQ10 can ameliorate some of the biochemical disturbances associated with CKD.
This approach offers a novel avenue for the management of CKD, highlighting the role of mitochondrial health in slowing the progression of kidney damage and improving the overall quality of life for patients.
Such interventions, if validated in larger clinical trials, could become part of the standard nutriceutical regimen for CKD, offering a non-invasive and potentially cost-effective treatment strategy.
Join us to end the kidney disease epidemic
Gut Microbiota's Role in IgA Nephropathy: Linking Diet, Immunity, and Kidney Health
In a study published in the Journal of Trace Elements in Medicine and Biology, researchers investigated the connection between gut microbiota and the production of galactose-deficient IgA1 (GdIgA1), a key factor in IgA nephropathy (IgAN).
The research, using 16S ribosomal RNA gene sequencing, identifies significant differences in the fecal microbiota of IgAN patients compared to healthy controls, notably an increased abundance of Escherichia-Shigella.
This bacterium correlates with elevated levels of GdIgA1 and specific immune responses. Additionally, the study observed a decrease in IgA protease-producing commensal bacteria in IgAN patients, which might contribute to the disease by altering immune regulation in the gut.
Why is this important?
Understanding the role of gut microbiota in the development of IgA nephropathy enhances our knowledge of the disease's pathogenesis and could lead to novel therapeutic strategies.
By elucidating the connection between diet, microbiota, and systemic immune responses, this research paves the way for targeted interventions that could modify the gut flora to reduce GdIgA1 production and potentially slow the progression of kidney disease.
This emphasizes the importance of a holistic approach in treating this kidney disease, considering a comprehensive gut restoration protocol to target its root cause.
Review article of the month
Interplay between periodontitis and chronic kidney disease
Periodontitis, a common chronic inflammatory condition affecting the tooth-supporting tissues, not only leads to tooth loss but also has significant systemic effects, including increasing the risk of chronic kidney disease (CKD).
This relationship between periodontitis and CKD, and other non-communicable diseases (NCDs) like diabetes and cardiovascular disease, appears to be bidirectional and independent of shared risk factors, suggesting that periodontitis acts as a non-traditional risk factor for these conditions.
The pathophysiology of periodontitis involves a dysregulated immune response to microbial dysbiosis in the periodontal area, leading to systemic inflammation that mirrors and potentially exacerbates CKD. Further research through large-scale intervention studies is necessary to establish a definitive causal link and could pave the way for new integrated treatment approaches for both conditions.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
January Research and News 2025
We delve deeply into numerous medical journals to bring you the latest insights on Integrative Medicine's impact on kidney health, recognizing the importance of your time. Our dedication to curating and concisely summarizing these essential studies continues unabated. Welcom to the January Research and News.
Optimal Timing for Chronic Kidney Disease Screening: A Cost-Effectiveness Perspective
This study conducted by Marika M. Cusick and colleagues assesses the cost-effectiveness of initiating chronic kidney disease (CKD) screening at various ages in conjunction with the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors.
Utilizing a decision-analytic Markov model, the research explored the health outcomes and financial implications of starting CKD screenings at ages ranging from 35 to 75 years, performed every 5 to 10 years.
The findings revealed that starting screenings every 5 years from age 55, combined with SGLT2 inhibitors, provides a cost of $128,400 per quality-adjusted life year (QALY) gained.
Although earlier screenings at ages 35 or 45 yielded greater health benefits, the costs exceeded $200,000 per QALY, surpassing typical cost-effectiveness thresholds.
Why Is This Important?
This analysis is pivotal for health policy development, suggesting that initiating CKD screening at age 55 with subsequent screenings every 5 years is the most cost-effective strategy when paired with modern treatments like SGLT2 inhibitors.
These findings can help optimize healthcare resource allocation, ensuring that interventions not only enhance patient outcomes but also represent a financially viable strategy.
Medication-Induced Acute Kidney Injury: Insights from a Cross-National Drug-Wide Association Study
Alessandro Bosi and colleagues conducted an exploratory Drug-Wide Association Study (DWAS) to identify medications linked with increased risk of acute kidney injury (AKI) using data from Denmark and Sweden between 1997 and 2021.
The study employed a case-time control design, examining drug dispensing patterns before AKI hospitalization. Conditional logistic regression adjusted for comorbidities estimated the association between drug exposure and AKI risk.
Key findings identified 16 medications linked to AKI, validated through pharmacovigilance data and literature reviews, including known risk medications like furosemide and penicillin, and others requiring further study like opioids.
Why is this Important?
Understanding the link between drug use and AKI is crucial for enhancing patient safety and optimizing medication management. By pinpointing potential risk factors, healthcare providers can better anticipate and mitigate AKI risks, especially in vulnerable populations.
Cross-Generational Effects of Maternal Low-Level Exposure to Air Pollution on Kidney Health
A study led by Hui Chen and colleagues, published in the American Journal of Nephrology, investigated the renal health effects of maternal exposure to low levels of PM2.5 particulate matter during the peri-pregnancy period.
Using female Balb/c mice exposed to PM2.5 before and during pregnancy, researchers found that while maternal kidneys showed increased oxidative stress without histological changes, male offspring experienced reduced body and kidney weights, lower glomerular counts, and significant renal damage post-birth.
Female offspring displayed delayed development and similar kidney impairments. Notably, discontinuing PM2.5 exposure from conception markedly reduced kidney damage in offspring, highlighting a critical window for mitigating pollution's adverse effects.
Why is this Important?
This research underscores the latent risks of even low-level air pollution on renal health across generations, emphasizing that there are no safe levels of PM2.5 exposure during critical developmental periods.
The findings illuminate the urgent need for stringent environmental controls and public health strategies to protect vulnerable populations, particularly during pregnancy, from ambient air pollution.
Moreover, the study provides valuable insights into the timing of exposure reduction to prevent or lessen chronic kidney disease in offspring, contributing to better guidelines and interventions for expecting mothers in regions with varying air quality levels.
Join us to end the kidney disease epidemic
Restricting Potassium Intake Linked to Faster Chronic Kidney Disease Progression
A study by Tatsuya Suenaga and colleagues, published in Nephrology Dialysis Transplantation, explored the association between potassium intake and the progression of chronic kidney disease (CKD) in a cohort of 4,314 Japanese patients tracked over five years.
Using the Tanaka formula to estimate potassium levels from urine samples, researchers found that lower potassium intake significantly correlated with accelerated CKD progression.
Specifically, patients in the lowest quartile of potassium intake had a higher risk of CKD advancement compared to those with higher intake levels. This relationship persisted even when adjusting for multiple variables, suggesting that maintaining adequate potassium intake could be crucial in managing CKD progression.
Why is this Important?
This study highlights the critical role of potassium intake in managing CKD progression, challenging the existing hesitance to recommend specific potassium levels due to fears of hyperkalemia.
The findings suggest that a balanced approach to potassium consumption could benefit patients with CKD, potentially delaying the onset of end-stage kidney disease.
This has significant implications for dietary recommendations and patient management in the CKD population, emphasizing the need for individualized dietary planning to optimize renal health while minimizing risks.
Review article of the month
Link Between Protein-Energy Wasting and the Progression of Chronic Kidney Disease
This review focuses on the significance of managing protein-energy wasting (PEW) in CKD patients not on dialysis to delay the progression of CKD and the initiation of dialysis. It highlights the correlation between PEW and poor clinical outcomes such as increased mortality and hospitalizations, driven by factors like low serum albumin, low BMI, and insufficient dietary intake of energy and protein.
The review suggests that optimizing nutrition through a moderately low protein and plant-dominant diet (PLADO), along with necessary supplementation during acute kidney injury, in conjunction with managing comorbidities and promoting exercise, could effectively prevent and treat PEW, thereby slowing CKD progression.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
Integrative Approach to Kidney Stones Prevention and Management
Kidney stones, also called nephrolithiasis or urolithiasis, are complex diseases influenced by multiple factors, including genetic and environmental factors. Stones are often painful and, left unaddressed, can lead to more serious conditions such as obstruction of the urinary tract and permanent damage to the kidneys. This blog post will discuss the integrative approach to kidney stones prevention and management.
By Majd Isreb, MD, FACP, FASN, IFMCP The frequency of kidney stones has been on the rise in the United States (US), according to nationally published data. The National Health and Nutrition Examination Survey (NHANES) has analyzed the health and nutrition status of the general population for the past 30 years. According to analyses from these publications, the lifetime chance of developing kidney stones in an adult (age 20-74) increased from 3.2% in the 1970s to 5.2% in the 1980s. The most recent survey using data between 2007 and 2010 showed that the lifetime chance for kidney stones in an adult is now up to 8.8%. That’s almost a three-fold increase over three decades.
In addition to the inconvenience and pain associated with kidney stones, they also pose a significant healthcare burden and cost. Patients with kidney stones are likely to present to the emergency department and are often hospitalized for an average of 2-3 days. If patients cannot pass them, they may require surgical intervention. In 2000, the total cost of caring for patients with kidney stones in the US was estimated at $2.1 billion. Furthermore, it is estimated that the cost of care will rise by $1.24 billion per year by 2030.
Kidney Stones: Types and Formation
Before discussing the integrative approach to kidney stones prevention and management, it is prudent to discuss the various types of kidney stones. There are five major types of kidney stones: calcium oxalate, calcium phosphate, uric acid, struvite (magnesium ammonium phosphate), and cystine. Calcium oxalate is by far the most common, comprising approximately 75% of kidney stones.
Calcium oxalate and calcium phosphate stones
Calcium stones are the most common type of kidney stones. They are composed of either calcium oxalate or calcium phosphate compounds. They are formed when calcium binds to oxalate (or phosphate) in the urine. On the other hand, dietary calcium can bind oxalate in the intestine and prevent its absorption through the gut, so there is less in the urine to form stones.
Oxalates are compounds found naturally in certain foods (nuts, spinach, potatoes, tea, and chocolate). In those prone to calcium oxalate formation, eating high amounts of foods rich in oxalates can increase the amount of oxalate in the urine and increase the risk of stone formation.
Calcium phosphate stones are less common than calcium oxalate stones. Causes include hyperparathyroidism (when the body produces too much parathyroid hormone), renal tubular acidosis (a kidney condition that causes a buildup of acid in the body), and urinary tract infections. It is important to understand if one of these conditions is behind the formation of calcium phosphate stones.
Inadequate hydration is a major risk factor for these types of stones. Certain medications can also reduce the risk of stone formation, including thiazide diuretics (for example, hydrochlorothiazide), which reduce calcium levels in the urine available to form stones. Potassium citrate binds to calcium, preventing it from binding to oxalate and phosphate to form stones.
Uric acid stones
Uric acid stones generally form when urine is too acidic, causing otherwise normal levels of uric acid to dissolve into the urine, where it may crystallize, forming stones. Therefore, by alkalizing the pH of the urine, we can prevent crystal formation.
In these cases, potassium citrate is the most common medication used to manage uric acid stone formation. Another medication, sodium bicarbonate, can also be used to alkalinize the urine.
However, in some individuals, consumption of too much animal protein can actually increase the production of uric acid. In these cases, dietary restriction of animal protein might be necessary to manage excessive uric acid production. Use of allopurinol, a medication that prevents uric acid formation from precursors xanthine and hypoxanthine, may also be indicated.
Struvite stones
Struvite stones are composed of magnesium ammonium phosphate and, unlike other stones, form in alkaline urine. Most commonly, these types of stones form due to a bacterial infection that raises the urine pH to alkaline levels. To dissolve these stones, acetohydroxamic acid (AHA) is used to reduce urine pH and ammonia.
Cystine stones (least common kidney stone type)
Cystine stone formation (also called cystinuria) is a relatively uncommon type of stone and the result of a genetic condition. As a result, urinary elevations of the amino acid cystine result in stone formation. Cystine stones can often be managed by improving hydration and maintaining alkaline urinary pH through diet and medication.
The Integrative Approach to Kidney Stones Treatment
Conventionally, the treatment approach may include a multi-pronged approach and may include medication, dietary and lifestyle, surgical removal, and using ultrasonic waves to break up stones.
There are a few conventional dietary guidelines, but guidelines tend to focus too far downstream, on stone composition, not on the underlying pathology and across risk factors upstream to prevent formation. By understanding the pathology and risk factors involved, we can better understand why some people develop stones while others do not and effectively reduce the incidence of urolithiasis.
Socioeconomic and Environmental factors that impact the development of kidney stones
When thinking about the integrative approach to kidney stones, it is important to understand the evironmental factors that impacts its development. Studies of the distribution of kidney stones in the US suggest that geography is an important consideration in risk. For example, individuals who live in southern states are more likely to have kidney stones than those who live in the North. In fact, inhabitants of the Southeast are nearly twice as likely to have a history of kidney stones as compared to those living in the Northwest. This has earned North Carolina, South Carolina, Georgia, Alabama, Mississippi, and Tennessee the nickname “the stone belt.”
Incidentally, these states also lead the nation in obesity and the incidence of diabetes. Inhabitants of these states are also more likely to consume a Standard American Diet (SAD), increasing the risk of pH imbalance that can lead to kidney disease and risk of stone formation.
Another factor to consider is climate. Higher ambient temperature and sunlight index are associated with a higher risk for kidney stones. Also, the incidence of kidney stones is higher in the summer than in the winter. This may partly explain why inhabitants of the warmer states in “the stone belt” have such a high incidence of kidney stone formation.
Why is this significant? Understanding this disproportionate distribution can help us understand the complexity of kidney stone pathology to better build a better approach. Our integrative approach to prevention and treatment should be sensitive to underlying socioeconomic and environmental contributions as well as factors that reduce access to adequate healthcare and good nutrition.
Impact of Diet on Kidney Stones
When the subject of urolithiasis and diet comes up, recommendations about the intake of calcium, oxalates, and hydration cannot be avoided. However, the reality is that we should be looking at broader considerations when it comes to the integrative approach to kidney stone prevention.
Consumption of the standard American diet (SAD) seems to increase the risk of kidney stone formation. SAD includes the consumption of sugary beverages and soda, as well as an elevated intake of processed foods and animal protein. Interestingly, eating more fresh produce is protective. This is associated with nutritional benefits, including (but not limited to) foods rich in potassium, magnesium, and fiber. Furthermore, taking vitamin D, and good hydration risk were reversed.
This topic deserves a deeper dive, and we do so in another blog [found here].
Genetics and Kidney Stones
There seems to be a familial link when it comes to the development of kidney stones. Recently, genome-wide association studies uncovered several genetic sequence variants (SNPs) that lead to an increased risk of kidney stone development. Single nucleotide polymorphisms (SNPs) have been associated with kidney stones, including those found in CLDN14, ALPL, SLC34A1, CASR, VDR, OPN, and TRPV5.
These genes play a role in the way the kidney handles certain vitamins and minerals, including vitamin D, calcium, and phosphate. Imbalances of these nutrients are involved in the pathophysiology of stone formation; therefore, it stands to reason that genetic variations that result in mishandling will increase the risk. There is still a lot more to learn about the contribution of genetic factors to stone formation. However, what we do know is that we can modulate these risks through environmental and dietary modification.
Microbiome and Kidney Stones
Balance of the gut bacteria also plays an important role in causing or preventing kidney stones. The most studied organism is Oxalobacter formigenes, which has been found to be protective when present in adequate quantities as part of the GI microflora. This bacterium degrades oxalate in the gut, decreasing its absorption and excretion in the urine.
In addition, dysbiosis, in general, is linked to kidney stone formation in those people prone to stone formation due to genetic or environmental factors. Therefore (and unsurprisingly), gut health is an important consideration when addressing kidney stones. Antibiotics which negatively alter the gut microbiome, are linked to higher rates of kidney stones.
More on the contribution of the microbiome to kidney stone formation will be the topic of another blog on the gut-kidney axis here.
The Bottom Line
Although genetic factors may impact the risk of kidney stone formation, environmental, dietary, as well as factors affecting the integrity of the gut microbiome, play a large role in turning on those genes and impacting stone formation. Therefore, the integrative approach to addressing kidney stones must account for a combination of all these factors, and practitioners should formulate a personalized approach that modifies relevant lifestyle factors.
This blog was written with contributions from Lara Zakaria, RPh MS CNS CDN IFMCP
The Anti-Inflammatory and Anti-Fibrotic Potential of Colchicine in Kidney Disease: A Deep Dive
Colchicine, a well-established medication primarily used to treat gout and familial Mediterranean fever, has drawn increasing attention for its anti-inflammatory and anti-fibrotic properties. In the context of kidney disease, where inflammation and fibrosis are significant contributors to disease progression, colchicine’s therapeutic potential is compelling. By targeting pathways central to inflammation and tissue remodeling, colchicine offers a novel approach to managing kidney disease while also raising considerations around metabolism, pharmacogenomics, and drug interactions. This blog discusses the role of colchicine in kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Anti-Inflammatory Properties of Colchicine in Kidney Disease
Colchicine exerts its anti-inflammatory effects by disrupting microtubule assembly, which impairs the activation and migration of neutrophils. This mechanism reduces the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β). By modulating the NLRP3 inflammasome—a critical driver of inflammation in various kidney conditions—colchicine effectively dampens inflammatory responses that could otherwise exacerbate renal injury.
In chronic kidney disease (CKD), inflammation plays a pivotal role in disease progression. Colchicine's ability to curb this inflammation has sparked interest in its use for CKD patients. In a study of greater than 3,000 CKD patients, Colchicine was found to slow the progression of CKD. Additionally, its potential to lower levels of systemic inflammation may benefit cardiovascular outcomes, a major concern in CKD patients.
Anti-Fibrotic Mechanisms and Implications of Colchicine in Kidney Disease
Fibrosis, characterized by excessive deposition of extracellular matrix proteins, is a hallmark of progressive kidney disease. This progressive scarring makes advanced CKD often irreversible. Colchicine's anti-fibrotic properties stem from its ability to inhibit fibroblast activation and disrupt collagen synthesis. These effects have been demonstrated in experimental models of renal fibrosis, where colchicine reduced scarring and preserved renal architecture.
Clinical evidence suggests that colchicine may help mitigate fibrosis in conditions like IgA nephropathy and diabetic kidney disease, both of which involve substantial inflammatory and fibrotic components. It has also been shown to decrease fibrosis in kidney transplant grafts. By reducing fibrosis, colchicine could preserve kidney function and delay the need for dialysis.
Metabolism and Pharmacogenomics of Colchicine
Understanding how colchicine is metabolized is critical for optimizing its use in kidney disease. Colchicine is primarily metabolized in the liver by the cytochrome P450 enzyme CYP3A4 and undergoes biliary and renal excretion. This dual elimination pathway makes colchicine a challenging drug for patients with impaired kidney function, as reduced clearance can lead to toxicity.
Pharmacogenomics plays a significant role in colchicine metabolism. Genetic polymorphisms in CYP3A4 and the P-glycoprotein transporter (encoded by the ABCB1 gene) can significantly alter drug levels, impacting efficacy and safety. For example, patients with reduced P-glycoprotein transporter activity may experience higher colchicine exposure, increasing the risk of adverse effects such as myopathy and gastrointestinal distress.
Incorporating pharmacogenomic testing into clinical practice could personalize colchicine therapy, ensuring appropriate dosing and minimizing the risk of toxicity, particularly in populations with a high prevalence of genetic variations.
Join us to end the kidney disease epidemic
Potential Drug Interactions and Safety Considerations
Colchicine is highly susceptible to drug-drug interactions due to its dependence on CYP3A4 and P-glycoprotein for metabolism and transport. Medications that inhibit these pathways, such as certain macrolide antibiotics, antifungals, and calcium channel blockers, can significantly increase colchicine levels and toxicity risk. Co-administration with these drugs necessitates careful dose adjustments or alternative therapies.
Moreover, colchicine's narrow therapeutic index demands vigilance in patients with kidney disease. Impaired renal function amplifies the risk of adverse effects, making dose reduction or extended dosing intervals essential. Physicians must also monitor for cumulative toxicity, particularly in elderly patients and those with concurrent hepatic impairment.
The following five common medications are known to interact with colchicine and using a lower dose in these patients is a must:
- Clarithromycin (and other macrolide antibiotics).
- Atorvastatin (and other statins).
- Verapamil (and other calcium channel blockers).
- Cyclosporine and tacrolimus.
- Ketoconazole (and other antifunguals).
Clinical Utility of Colchicine in Kidney Disease
Emerging evidence supports colchicine’s clinical utility in specific kidney disease contexts. In patients with gout and CKD, colchicine offers a safer alternative to NSAIDs for managing acute flares. It has also shown promise in reducing proteinuria and stabilizing renal function in small studies of IgA nephropathy.
In the realm of acute kidney injury (AKI), colchicine's ability to modulate inflammation may offer protective benefits. Ongoing trials aim to elucidate its role in preventing AKI in high-risk settings, such as cardiac surgery and sepsis. Furthermore, colchicine’s cardiovascular benefits, demonstrated in conditions like pericarditis and coronary artery disease, are particularly relevant for CKD patients at heightened cardiovascular risk.
It is important to remember though that AKI has been reported with the use of high doses of colchicine.
The Bottom Line on Colchicine in Kidney Disease
Colchicine’s anti-inflammatory and anti-fibrotic properties position it as a promising therapeutic option in kidney disease management. However, its clinical utility must be balanced against the risks of toxicity, particularly in populations with impaired renal function or pharmacogenomic predispositions. Personalized approaches, including pharmacogenomic testing and careful monitoring of drug interactions, will be essential to unlocking colchicine's full potential in nephrology.
December Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the December Research and News.
Dietary Patterns and Kidney Health: Insights from the CHRIS Study Using Reduced Rank Regression
The CHRIS study, involving 8,686 participants, utilized reduced rank regression (RRR) to derive dietary patterns (DPs) based on nutrient intake and assess their association with kidney health.
Split into groups with and without kidney disease, hypertension, or diabetes, the study identified three distinct DPs reflecting various nutrient levels.
Analysis revealed complex associations between these DPs and kidney outcomes, such as creatinine-based eGFR and microalbuminuria, which varied by health status and sex.
This approach highlighted the significant influence of combined dietary nutrients on kidney health compared to single nutrients alone.
Why Is This Important?
Understanding the impact of specific dietary patterns on kidney health can guide nutritional interventions for preventing or managing chronic kidney diseases (CKD), especially in populations with existing health conditions.
This study underscores the potential of tailored dietary strategies to enhance kidney function and prevent disease progression in individuals at risk due to conditions like diabetes and hypertension.
This study is available for full access and is free to download.
Acetylcholine Production in Podocytes: A Protective Mechanism Against Kidney Injury in Glomerulonephritis
Research from the Journal of the American Society of Nephrology identifies that podocytes, specialized cells in the kidneys, produce acetylcholine via the enzyme choline acetyltransferase (ChAT), previously thought to be limited mainly to neuronal cells.
Utilizing ChAT transgenic mice, the study demonstrated that acetylcholine production in podocytes significantly mitigated damage in models of antiglomerular basement membrane glomerulonephritis (anti-GBM GN).
This protection was evident in the substantial reductions in glomerular proliferation, fibrinoid necrosis, and tubular injury.
The protective mechanism of acetylcholine involves diminishing inflammation, supporting angiogenic factors, and boosting endothelial nitric oxide synthase expression.
Why Is This Important?
This study underscores a novel non-neuronal role for acetylcholine in protecting against kidney injury, suggesting that enhancing acetylcholine production within the kidneys could be a viable therapeutic strategy.
Natural methods to boost acetylcholine include dietary choices rich in choline, such as eggs, liver, and soybeans. Additionally, stimulating the vagus nerve, which releases acetylcholine, could indirectly support systemic levels of this neurotransmitter and may enhance its renal protective effects.
This mechanism offers the potential for non-pharmacological strategies to complement medical treatments for kidney diseases, potentially improving outcomes through lifestyle modifications that increase acetylcholine levels.
Causal Link Between Diet and IgA Nephropathy: Insights from a Mendelian Randomization Study
This Mendelian randomization study utilized genome-wide association data to explore the causal effects of various dietary factors on immunoglobulin A nephropathy (IgAN).
Researchers analyzed the relationship between 26 dietary exposures and IgAN using multiple analytical methods. The findings revealed that frequent alcohol consumption increases the risk of IgAN, while consuming cheese, cereals, and sushi offers protective benefits.
The study highlights specific dietary interventions that may influence the development and progression of IgAN, providing a genetic basis for dietary recommendations in managing this condition.
Why Is This Important?
Understanding the dietary influences on IgAN can lead to targeted nutritional guidelines that potentially prevent or mitigate the disease's progression.
This study's genetic evidence offers a more reliable assessment of the causal relationships between diet and IgAN, moving beyond mere associations to suggest practical interventions.
These findings could lead to personalized dietary strategies that enhance treatment outcomes for individuals with IgAN, emphasizing the importance of dietary choices in managing kidney health.
Join us to end the kidney disease epidemic
Adding Five Minutes Of Exercise To Daily Routine Lowers Blood Pressure, Cuts the Odds For Heart Disease
This cross-sectional study from the Prospective Physical Activity, Sitting, and Sleep Consortium (ProPASS) examined the relationship between daily activities and blood pressure (BP) among 14,761 participants.
Utilizing thigh-worn accelerometers, the study categorized 24-hour behavior into six parts: sleeping, sedentary behavior, standing, slow walking, fast walking, and exercise-like activities such as running and cycling.
Findings indicate that additional time spent in exercise-like activities, even as little as five minutes, is linked to reductions in both systolic and diastolic blood pressure.
Conversely, increased sedentary time was associated with higher BP, while standing and walking showed minimal impact.
Why Is This Important?
Understanding the influence of everyday physical activities on blood pressure can lead to more effective lifestyle interventions for hypertension management.
This study highlights the significant benefits of incorporating brief periods of exercise into daily routines, offering a practical approach to reducing blood pressure levels in the general population.
Promoting small, manageable increments of physical activity could serve as a feasible public health strategy to combat hypertension, emphasizing the critical role of active living for cardiovascular health.
Review article of the month
Genetic Testing in Adults with Kidney Disease
A systematic review and meta-analysis published in the Clinical Journal of the American Society of Nephrology evaluates the diagnostic utility of genetic testing in adults with chronic kidney disease (CKD). The study, which included data from 60 studies and 10,107 adults with CKD, revealed a significant diagnostic yield of 40% from genetic testing, with variability depending on CKD subtype, highest at 62% for cystic kidney diseases.
Key findings also include the utility of genetic testing in reclassifying diagnoses and informing treatment changes and family screening, demonstrating that genetic testing can substantially aid in pinpointing the underlying causes of CKD, particularly when traditional diagnostic methods fall short.
Despite the promising results, the study noted limitations such as heterogeneity across studies regarding testing techniques and patient characteristics.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you would like us to focus on specific topics. Please email us at info@inkidney.com.
Top 10 Kidney-Friendly Superfoods: Science Meets Holistic Nutrition
Your kidneys work tirelessly to filter waste, balance electrolytes, and keep your body functioning smoothly. Supporting them with the proper nutrition is essential, especially if you're managing chronic kidney disease (CKD) or want to maintain optimal kidney health. Enter kidney-friendly superfoods: scientifically-backed powerhouses that can reduce oxidative stress, combat inflammation, and support filtration. Let’s explore the top 10 foods and how you can incorporate them into your meals.
By Majd Isreb, MD, FACP, FASN, IFMCP
The Top 10 Kidney-Friendly Superfoods
Blueberries
These little berries pack a punch with anthocyanins, powerful antioxidants that combat oxidative stress and reduce inflammation. Plus, they’re low in potassium, making them a go-to snack and true kidney-friendly superfoods.
- Recipe Idea: Blend blueberries with spinach and unsweetened almond milk for a refreshing smoothie.
Red Bell Peppers
Vibrant red bell peppers are rich in vitamins A and C and low in potassium. These nutrients support immunity and reduce inflammation, both critical for kidney health. Furthermore, red bell peppers and chili peppers contain capsaicin, which was found to prevent acute kidney injury, slow the progression of diabetic and chronic kidney disease, ameliorate hypertension, and delay renal cancer growth.
- Recipe Idea: Roast red bell peppers and blend them into a creamy cauliflower soup.
Cauliflower
A versatile vegetable, cauliflower is high in fiber and low in potassium. Cauliflower and other cruciferous vegetables (broccoli and Brussels sprouts) contain glucosinolates, which help detoxify the body and aid digestion, reducing the buildup of harmful toxins.
- Recipe Idea: Swap rice for cauliflower rice in your stir-fry for a kidney-friendly alternative.
Garlic
Garlic isn’t just a flavor booster—it’s an anti-inflammatory superstar that helps lower cholesterol and reduces sodium reliance. These antioxidant and anti-inflammatory properties are the result of its constituent organosulfur compounds. Garlic supplements were found to be effective in slowing diabetic kidney disease.
- Recipe Idea: Toss roasted garlic and herbs with your favorite vegetables for a savory side dish.
Cranberries
Known for their role in preventing urinary tract infections (UTIs), cranberries also contain antioxidants that support kidney health. They (along with other wild berries) have been found to be an excellent therapeutic option for uremic dysbiosis in chronic kidney disease.
- Recipe Idea: Make a cranberry chia seed pudding for a sweet and tangy dessert.
Onions
Onions are packed with quercetin, a flavonoid that reduces inflammation. They’re also a great way to add flavor without extra salt. Onion has also been found to prevent cadmium-induced kidney injury.
- Recipe Idea: Sauté onions with zucchini for a quick and flavorful side dish.
-
Join us to end the kidney disease epidemic
Egg Whites
Egg whites provide high-quality protein without the phosphorus found in egg yolks. While the whole egg may be beneficial, there are some concerns about the concentration of phosphorus and the trimethylamine N-oxide precursor, choline, in egg yolks. This makes egg whites perfect for maintaining protein intake without straining the kidneys.
- Recipe Idea: Whip up a spinach and egg white omelet for a hearty breakfast.
Cabbage
Low in potassium and phosphorus, cabbage is another cruciferous vegetable that is full of fiber and phytonutrients that support detoxification. Studies on cabbage juice found that it modulates phase II biotransformation (detoxification) enzymes. It was also found to protect against lead-induced kidney damage. Additionally, red cabbage can improve blood pressure and kidney function in diabetic kidney disease.
- Recipe Idea: Toss shredded cabbage with apple slices and a lemon dressing for a crunchy slaw.
Apples
Apples are rich in pectin, a soluble fiber that helps lower cholesterol and toxins. Their anti-inflammatory properties make them a staple for kidney health. In addition to its fiber and antioxidant content, apples as fruits can lower dietary acid load and protect the kidneys. This led some authors to say, "An Apple a Day Keeps Dialysis Away." Apples are truly kidney-friendly superfoods.
- Recipe Idea: Bake apple slices with cinnamon for a cozy and healthy treat.
Olive Oil
Packed with anti-inflammatory compounds and heart-healthy fats, olive oil is a significant part of the Mediterranean diet that was found to slow the progression of kidney disease. Furthermore, olive oil provides energy without overloading the kidneys.
- Recipe Idea: Drizzle olive oil and lemon over a fresh salad or grilled vegetables.
How These Kidney-Friendly Superfoods Support Kidney Health
- Reducing Oxidative Stress: Antioxidants in foods like blueberries, cranberries, and red bell peppers help neutralize harmful free radicals that can damage kidney tissues.
- Lowering Inflammation: Garlic, onions, and olive oil contain anti-inflammatory compounds that protect the kidneys and reduce the risk of chronic damage.
- Supporting Filtration: High-fiber foods like apples and cauliflower aid in the intestinal removal of uremic toxins and ease the kidneys' workload.
Recipe Ideas to Bring All Kidney-friendly Superfoods Together
Superfood Salad Bowl
Combine shredded cabbage, spinach, diced apples, and roasted red peppers. Drizzle with olive oil and a squeeze of lemon juice for a simple, nutrient-packed meal.
Kidney-Friendly Breakfast Smoothie
Blend a handful of blueberries, unsweetened almond milk, and a scoop of spinach for a refreshing and energizing start to your day.
Hearty Vegetable Stir-Fry
Sauté cauliflower rice, garlic, onions, and red bell peppers in olive oil. Add a dash of herbs for a flavorful and kidney-friendly dish.
Join us to end the kidney disease epidemic
The Bottom Line on Kidney-Friendly Superfood
Your kidneys deserve the best care, and incorporating these superfoods into your diet is a delicious way to support their health. Remember, every individual’s nutritional needs are unique, so consult your Integrative healthcare provider or dietitian before making significant changes.
What’s your favorite kidney-friendly superfood? Share your recipe ideas or tips with us at info@inkidney.com —we’d love to hear from you!
Your Guide to Avoiding Glyphosate Exposure
Glyphosate, the active ingredient in a widely used herbicide, is under increasing scrutiny for its potential impact on health. While its link to cancer has been widely discussed, emerging research suggests that glyphosate exposure may also contribute to kidney disease. This is a critical issue given the rising prevalence of kidney-related conditions worldwide.
Glyphosate is an agricultural chemical that often contaminates food. Studies have found alarmingly that some popular foods, such as cereals, granola, and hummus, contain glyphosate levels far exceeding organic alternatives. Understanding the potential risks of glyphosate exposure and adopting strategies to reduce it can significantly improve kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Why Glyphosate Exposure Matters
Glyphosate is pervasive. It is often sprayed on crops to dry them out before harvest. This process, known as desiccation, allows for quicker and more efficient harvesting but leads to higher glyphosate levels in our food. Commonly contaminated foods include oat-based cereals, granola bars, and even hummus. Conventional oat-based cereals, for example, have been found to contain glyphosate levels up to 700 times higher than their organic counterparts.
This matters because chronic exposure to glyphosate, even in small amounts, can have significant health implications. For kidney health specifically, the chemical’s impact is tied to oxidative stress, inflammation, and changes in gut microbiota—all of which can worsen or trigger kidney dysfunction.
Glyphosate Exposure and Kidney Disease: The Evidence
Emerging research highlights several ways in which glyphosate exposure may harm kidney health:
- Oxidative Stress: Glyphosate can promote the generation of free radicals in the body. These unstable molecules damage cells and tissues, contributing to chronic kidney conditions over time.
- Gut Microbiota Disruption: Glyphosate acts as an antimicrobial agent, altering the balance of beneficial gut bacteria. An unhealthy gut microbiome has been linked to the progression of chronic kidney disease (CKD) through systemic inflammation and impaired metabolic processes.
- Cumulative Toxicity: Over time, the kidneys, which filter toxins from the bloodstream, may accumulate glyphosate and other environmental contaminants. This build-up can lead to conditions such as tubulointerstitial nephritis, a form of kidney inflammation.
- Genetic Susceptibility: While more research is needed, early studies suggest that individuals with specific genetic vulnerabilities may be at higher risk of glyphosate-induced kidney damage.
This blog contains much more details about the effects of glyphosate exposure on kidney health.
Organic vs. Conventional Foods: A Stark Contrast
The Environmental Working Group (EWG) has conducted extensive testing to reveal the disparities in glyphosate levels between organic and conventional foods:
- Cereals: Conventional oat-based cereals contained an average of 711 parts per billion (ppb) of glyphosate, while organic cereals showed no detectable levels.
- Granola: Conventional granola samples averaged 298 ppb, compared to organic alternatives with no detectable glyphosate.
- Hummus: Conventional hummus showed 296 ppb, while organic hummus averaged 65 ppb.
While organic foods are not entirely immune to glyphosate contamination—likely due to pesticide drift from nearby fields—they remain a safer choice for reducing exposure.
Join us to end the kidney disease epidemic
How Glyphosate Exposure Impacts Broader Health
Beyond its effects on kidney health, glyphosate exposure has been linked to other serious health issues, including:
- Cancer Risk: The International Agency for Research on Cancer (IARC) has classified glyphosate as a probable carcinogen.
- Hormonal Disruption: Some studies suggest that glyphosate may interfere with endocrine function, affecting hormones critical for metabolism and overall health.
- Neurotoxicity: Long-term glyphosate exposure may contribute to neurological issues such as memory loss and impaired cognitive function.
Given these risks, it is essential to minimize glyphosate exposure whenever possible—not just for kidney health but for overall well-being.
How to Reduce Glyphosate Exposure and Protect Kidney Health
Here are actionable steps to reduce your glyphosate exposure and promote kidney health:
- Choose Organic Foods: Opt for organic cereals, granola, and hummus whenever possible. While not entirely glyphosate-free, organic options consistently show lower levels of contamination.
- Wash and Peel Produce: Though not foolproof, thoroughly washing and peeling fruits and vegetables can help reduce surface pesticide residues.
- Adopt a Detox-Friendly Diet: Include foods rich in antioxidants, such as berries, leafy greens, and nuts, to combat oxidative stress and support kidney health.
- Focus on Gut Health: A healthy gut microbiome is critical in preventing chronic kidney disease. Consider probiotics, fiber-rich foods, and reducing processed sugar intake to maintain a balanced gut.
- Support Policy Changes: Advocate for stricter regulations on glyphosate use, particularly for pre-harvest applications that contribute to high contamination rates.
- Stay Informed: Stay updated on research linking environmental toxins to kidney health. Awareness is a powerful tool for making informed decisions.
The Bottom Line on Avoiding Glyphosate Exposure
The connection between glyphosate exposure and kidney disease underscores the importance of addressing environmental factors in health care. While systemic changes are necessary to reduce glyphosate contamination in our food supply, individual actions—like choosing organic foods and supporting kidney-friendly dietary practices—can significantly reduce the risk.
By taking proactive steps, we not only protect our kidneys but also support our overall health in the face of widespread environmental challenges.
For more tips and insights into integrative approaches to kidney health, explore our blog regularly.
Understanding the Oxalobiome: A Key Player in Kidney Stone Formation
The microbiome is the collective genomes of microbes (bacteria, bacteriophage, fungi, protozoa, and viruses) that live inside and on the human body. The oxalobiome, on the other hand, is the collection of gut microbes involved in oxalate metabolism. It plays a significant role in kidney stone formation. High oxalate levels in the urine can contribute to the formation of calcium oxalate stones, the most common type of kidney stones. This blog will explore the oxalobiome, how it functions, and its impact on kidney stone development.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is the Oxalobiome?
The oxalobiome comprises specific gut bacteria capable of breaking down dietary oxalate. The most notable of these bacteria include Oxalobacter formigenes, which utilizes oxalate as its energy source, reducing the amount absorbed into the bloodstream and subsequently excreted by the kidneys. Other members of the oxalobiome include Lactobacillus sp., Bifidobacterium sp., and Escherichia Coli. Understanding the composition and health of the oxalobiome is essential for assessing its role in preventing kidney stones.
Bowel Disease and Kidney Stone Formation
Research has shown that individuals with bowel diseases, such as inflammatory bowel disease (IBD), Crohn’s disease, and ulcerative colitis, have a higher risk of developing kidney stones. This connection is primarily due to fat malabsorption and changes in gut function that accompany these conditions.
In cases of chronic bowel inflammation, the absorption of fat can be impaired. Excess fat in the gut binds dietary calcium, making it less available to bind dietary oxalate. This leads to higher levels of unbound oxalate in the intestines. The excess oxalate can then be easily absorbed into the bloodstream and filtered by the kidneys, where it can bind with calcium to form calcium oxalate stones.
Additionally, chronic diarrhea, often associated with these bowel diseases, can lead to dehydration and reduced urine volume, further increasing the risk of kidney stone formation. Finally, the disruption in gut microbiota (dysbiosis) due to inflammation or medication use can also alter the balance of the oxalobiome, diminishing the body's ability to metabolize dietary oxalate effectively and contributing to an increased risk of stone formation.
Antibiotic Exposure and Kidney Stone Formation
The use of antibiotics has been thought to increase the risk of kidney stone formation due to their impact on the gut microbiome. Antibiotics can disrupt the balance of beneficial bacteria in the gut, including those that form the oxalobiome, which is responsible for breaking down and metabolizing dietary oxalate.
In a study of 13 million children and adults from 1994 to 2015 in the United Kingdom, The Health Improvement Network (THIN) matched 25,981 patients with kidney stones to 259,797 controls and found an association between 12 classes of oral antibiotics and kidney stones. Sulfa-containing antibiotics had the highest risk for kidney stones.
Long-term or repeated courses of antibiotics can exacerbate this effect, making individuals more prone to kidney stone development. Additionally, the impact on gut health and the microbiome can persist for an extended period after antibiotic use, suggesting a lasting vulnerability to stone formation that should be considered in both medical and dietary planning.
It is essential to mention, though, that a recent study found that the perceived increased risk of symptomatic kidney stones with antibiotic use may be mainly due to both comorbidities and the prescription of antibiotics for urinary symptoms.
Join us to end the kidney disease epidemic
The Link Between Oxalobiome and Kidney Stones
When the gut's oxalate-degrading bacteria are compromised, dietary oxalate absorption increases, elevating urinary oxalate levels. High urinary oxalate can bind calcium and crystallize to form calcium oxalate stones. Factors that disrupt the oxalobiome include antibiotic use, poor dietary habits, and gastrointestinal conditions that alter gut flora.
Studies have demonstrated that kidney stone formers have microbiome profiles different from those of non-stone formers. Stone formers exhibited lower fecal microbial diversity than controls. Three microbes, Faecalibacterium, Enterobacter, and Dorea, were significantly less represented in fecal samples of stone formers. Interestingly, the abundance of Oxalobacter was not different between the groups in this study.
The Effect of Oxalobiome on Urinary Citrate
Urinary citrate prevents kidney stone formation by acting as a natural inhibitor of stone development. Citrate binds to calcium in the urine, reducing the amount of free calcium available to form calcium oxalate or calcium phosphate stones. Additionally, citrate helps maintain an alkaline urine pH, further inhibiting the crystallization of stone-forming minerals.
Studies have shown that higher urinary citrate levels are associated with a reduced risk of kidney stones. Low citrate levels, a condition known as hypocitraturia, can be influenced by various factors, including diet, metabolic conditions, and certain medications. Increasing dietary intake of citrate-rich foods, such as citrus fruits, or using citrate supplements may help raise urinary citrate levels and thus provide a protective effect against kidney stones.
It appears that the oxalobiome also plays a role in increasing urinary citrate to decrease the risk of kidney stone formation further. In fact, urinary citrate increased by 31% from baseline at 1 week after fecal transplantation. Urinary calcium and oxalate also decreased significantly. Another study confirmed this.
The Role of Short-Chain Fatty Acids
A healthy gut microbiome can produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, through the fermentation of dietary fibers. These SCFAs play a vital role in maintaining gut health and influencing various physiological processes.
SCFAs have been shown to modulate the intestinal absorption of oxalate through their impact on gut permeability and the regulation of ion transport. By promoting a healthy gut barrier, SCFAs can reduce the passive paracellular absorption of oxalate (in between the cells), limiting its entry into the bloodstream. See the figure below.
Additionally, SCFAs can influence the expression of transport proteins involved in active cellular oxalate absorption, potentially decreasing the amount of oxalate that passes into the systemic circulation. This regulatory effect helps to maintain balanced oxalate levels, reducing the burden on the kidneys and lowering the risk of oxalate crystal formation, which is crucial for preventing kidney stones.
The production of SCFAs and their beneficial actions highlight the importance of a diverse and well-functioning gut microbiome for supporting kidney health and mitigating stone risk.
Join us to end the kidney disease epidemic
Can We Manipulate the Oxalobiome to Prevent Stones?
Probiotics and prebiotics may support a healthy oxalobiome, though more research is needed to confirm their efficacy in preventing kidney stones. Potentially, boosting Oxalobacter formigenes and other beneficial bacteria could be a therapeutic approach to managing kidney stone risk in susceptible individuals. However, studies on using probiotics to prevent kidney stones have been disappointing. This is likely due to the temporary effect of short-term probiotics on gut microbial diversity.
Integrative Tips for Supporting Oxalate Metabolism and Kidney Health
- Limit High-Oxalate Foods: Moderation is key; consider consulting with a healthcare provider for a diet tailored to kidney stone prevention. Remember that most urinary oxalate is actually produced inside the body by the liver from collagen metabolism.
- Hydration: Drinking plenty of water helps flush out oxalate before it can contribute to stone formation.
- Pre and Probiotics: Including prebiotic-rich foods such as fiber and resistant starch and probiotic-rich foods like kefir and sauerkraut may support overall gut health and, indirectly, the oxalobiome.
The Bottom Line on Oxalobiome and Kidney Stone Formation
The intricate link between gut health and kidney stone formation highlights the vital role of a balanced microbiome in maintaining kidney health. The oxalobiome, responsible for metabolizing dietary oxalate, underscores how specific gut bacteria can prevent excess oxalate absorption, ultimately reducing kidney stone risk. Factors such as antibiotic use, bowel diseases, and dietary habits can disrupt the balance of these beneficial bacteria, contributing to an increased likelihood of stone formation.
Supporting a healthy gut through dietary choices rich in both probiotics and prebiotics can be a powerful preventive strategy. Incorporating foods such as kefir, sauerkraut, chicory root, and garlic can help maintain a diverse and balanced gut environment. These dietary habits, coupled with hydration, an alkaline diet rich in citrate, and moderation of high-oxalate foods, offer an integrative approach to managing kidney stone risk.
November Research and News 2024
As we delve deep into countless medical journals to uncover the latest on Integrative Medicine's approach to kidney health, we are always reminded of the value of your time. Our commitment remains steadfast in curating and succinctly summarizing these vital studies for you. Welcome to the November Research and News.
Fish Oil Supplementation and Chronic Kidney Disease Risk Reduction in Diabetic Patients
A study involving 24,497 diabetic patients from the UK Biobank explored the relationship between fish oil supplementation and the risk of developing chronic kidney disease (CKD).
Utilizing Cox proportional hazards regression models and mediation analysis, the study found that fish oil use was significantly associated with a reduced incidence of CKD, with a hazard ratio of 0.90.
This protective effect was partially mediated through improvements in glycolipid and inflammatory biomarkers such as HbA1c, C-reactive protein (CRP), and high-density lipoprotein cholesterol (HDL-C).
Notably, fish oil supplementation delayed the onset of CKD by approximately 2.79 years compared to non-users.
Why Is This Important?
The study highlights the potential of fish oil supplementation as a preventive strategy against CKD in diabetic patients, underscoring its benefits beyond cardiovascular health.
The mediation by key biomarkers offers insights into the biological pathways through which fish oil may exert its protective effects. These findings support initiatives promoting fish oil supplementation to mitigate the risk of kidney disease in patients with diabetes, thereby enhancing their overall prognosis and quality of life.
Omega 3 Fatty Acids Mitigate Transition from Acute Kidney Injury to Chronic Kidney Disease and Renal Fibrosis
A study published in Kidney360 evaluated the impact of omega-3 polyunsaturated fatty acids (ω3PUFAs) on the progression from acute kidney injury (AKI) to chronic kidney disease (CKD) and renal fibrosis using two mouse models.
The research demonstrated that mice fed with linseed oil, rich in ω3PUFAs, exhibited improved survival rates, reduced renal tissue damage, and less fibrosis compared to those fed with soybean oil, which is low in ω3PUFAs.
Key findings revealed that specific metabolites of eicosapentaenoic acid (EPA)—18-hydroxyeicosapentaenoic acid, 17,18-epoxyeicosatetraenoic acid, and 17,18-dihydroxyeicosatetraenoic acid—possess significant antifibrotic properties, effectively suppressing fibrotic markers in renal fibroblast cells.
Why Is This Important?
This research underscores the potential of ω3PUFAs, particularly EPA and its metabolites, as therapeutic agents in preventing the deterioration from AKI to CKD, a critical transition that significantly impacts patient morbidity and mortality.
By elucidating the antifibrotic effects of these fatty acids, the study provides a biochemical basis for considering dietary interventions with ω3PUFAs to manage or mitigate renal fibrosis and chronic kidney disease progression.
Impact of Air Pollution on Primary Glomerular Disease Progression
A study featured in Kidney International Reports investigated the influence of air pollution on the progression of primary glomerular diseases.
Utilizing data from the Nephrotic Syndrome Study Network (NEPTUNE) and CureGlomerulonephropathy (CureGN), researchers analyzed the effects of air pollutants—specifically particulate matter ≤2.5 μm (PM2.5), black carbon (BC), and sulfate—on kidney disease outcomes.
The study, which included participants with over two years of follow-up, employed Cox proportional hazards models to link pollution exposure with significant declines in kidney function or outright kidney failure.
Findings indicated that exposure to PM2.5 and BC significantly correlated with accelerated disease progression. Furthermore, elevated sulfate levels were strongly associated with higher serum levels of inflammation markers like TNF and interleukin-1β, and heightened activity in inflammatory pathways within the kidney.
Why Is This Important?
This research highlights the critical role environmental pollutants play in exacerbating kidney disease, particularly in vulnerable populations with existing renal conditions.
By establishing a clear link between specific air pollutants and the rapid progression of glomerular diseases, the study underscores the need for targeted public health strategies to reduce exposure to harmful airborne substances.
This could significantly aid in slowing disease progression in patients with primary glomerulopathies, ultimately improving outcomes and quality of life.
Join us to end the kidney disease epidemic
Impact of Sleep Disruption on Kidney Cyst Growth in Autosomal Dominant Polycystic Kidney Disease
The study published in the Journal of the American Society of Nephrology examines the effect of circadian clock disruption on the progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD).
Utilizing both human nephrectomy samples and a Pkd1RC/RC mouse model with the Bmal1 gene deletion, the research highlights significant alterations in clock gene expression, lipid metabolism, and increased cyst growth, cell proliferation, apoptosis, and fibrosis.
These findings suggest that disruptions in circadian rhythms, specifically through the deletion of the Bmal1 gene in renal collecting ducts, exacerbate ADPKD progression by influencing lipid metabolism and cellular processes.
Why Is This Important?
This research underscores the significant role of sleep in the progression of ADPKD. By linking circadian clock disruption directly to accelerated disease progression via changes in lipid metabolism and cellular proliferation, the findings open new avenues for strategies that could target sleep to slow or potentially reverse the growth of kidney cysts in ADPKD patients.
Review article of the month
Genetic Testing in Adults with Kidney Disease
A systematic review and meta-analysis published in the Clinical Journal of the American Society of Nephrology evaluates the diagnostic utility of genetic testing in adults with chronic kidney disease (CKD). The study, which included data from 60 studies and 10,107 adults with CKD, revealed a significant diagnostic yield of 40% from genetic testing, with variability depending on CKD subtype, highest at 62% for cystic kidney diseases.
Key findings also include the utility of genetic testing in reclassifying diagnoses and informing treatment changes and family screening, demonstrating that genetic testing can substantially aid in pinpointing the underlying causes of CKD, particularly when traditional diagnostic methods fall short.
Despite the promising results, the study noted limitations such as heterogeneity across studies regarding testing techniques and patient characteristics.
You can download the full PDF here.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Tubulointerstitial Nephritis (TIN): A Comprehensive Overview
Tubulointerstitial nephritis (TIN) is a kidney disorder characterized by inflammation of the tubules and interstitial tissue surrounding them. This inflammation can impair kidney function, leading to various degrees of kidney dysfunction or, in severe cases, kidney failure. The causes of TIN are diverse, ranging from infections and autoimmune diseases to drug reactions and genetic factors. Understanding the various triggers and mechanisms of TIN is crucial for prevention, diagnosis, and treatment. Moreover, adopting an integrative approach can help manage TIN holistically by addressing underlying causes and supporting kidney health through lifestyle, dietary, and therapeutic measures.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is Tubulointerstitial Nephritis?
Tubulointerstitial nephritis primarily affects the kidney’s tubules and the surrounding interstitium (the space between tubules). It differs from glomerulonephritis, which mainly affects the glomeruli, the filtering units of the kidney. In TIN, the inflammation can cause damage to the kidney tissues, leading to impaired reabsorption and secretion of various substances essential for the body. Symptoms of TIN may include fatigue, nausea, high blood pressure, and reduced urine output. In some cases, it can progress to chronic kidney disease (CKD) if not treated promptly.
TIN can be classified into acute and chronic forms. Acute TIN develops suddenly and is often reversible if diagnosed early and treated appropriately, while chronic TIN progresses more slowly and may lead to irreversible kidney damage.
Genetic Tubulointerstitial Nephritis
Although less common, genetic factors can play a role in the development of tubulointerstitial nephritis. Genetic TIN is often associated with hereditary conditions that lead to kidney dysfunction. These can include disorders such as medullary cystic kidney disease or autosomal dominant tubulointerstitial kidney disease (ADTKD).
ADTKD is a genetic disorder characterized by progressive kidney damage, leading to chronic tubulointerstitial nephritis. Mutations in specific genes like UMOD, MUC1, and REN are often responsible for the disease. Patients with genetic TIN may experience symptoms similar to other forms of TIN but often present with a family history of kidney disease and progressive decline in kidney function starting in adolescence or early adulthood.
Early detection and genetic testing can help in managing genetic TIN. Integrating genetic counseling into the care of individuals with a family history of kidney disease may provide valuable insights into their risk and guide treatment decisions.
Drug-Induced Tubulointerstitial Nephritis
One of the most common causes of acute tubulointerstitial nephritis is drug reactions. Medications can trigger an immune-mediated inflammatory response in the kidneys, leading to damage. Identifying the culprit drug and discontinuing its use is essential to halt the progression of TIN.
Join us to end the kidney disease epidemic
Medications That Can Cause TIN
The following table lists common medications associated with drug-induced TIN:
| Drug Class | Examples |
|---|---|
| Antibiotics | Penicillins, cephalosporins, rifampin |
| Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) | Ibuprofen, naproxen |
| Proton Pump Inhibitors (PPIs) | Omeprazole, esomeprazole |
| Diuretics | Furosemide, thiazides |
| Antivirals | Acyclovir, indinavir |
| Anticonvulsants | Phenytoin, carbamazepine |
| Immune Checkpoint Inhibitors | Nivolumab, pembrolizumab |
In cases of drug-induced TIN, discontinuing the offending drug usually leads to recovery, though steroids or other immunosuppressive treatments may be required in severe cases. Kidney function should be monitored closely to prevent long-term damage.
Supplements and Tubulointerstitial Nephritis
While medications are a well-known cause of TIN, certain supplements and herbal products can also trigger this condition. Herbal remedies, often perceived as natural and safe, may contain nephrotoxic compounds that can damage kidney tissues.
Supplements That Can Cause TIN
Some supplements and herbs have been associated with kidney damage, including TIN:
- Aristolochic acid (found in some traditional Chinese medicines) has been linked to severe kidney injury, including TIN.
- Chlorella is a dietary supplement made from green algae and has various uses. Acute TIN has been reported to be caused by the use of chlorella.
Patients with existing kidney conditions or those taking nephrotoxic medications should exercise caution when using supplements, as they may increase the risk of developing TIN.
Infection-Associated Tubulointerstitial Nephritis
Infections are the second most common cause of tubulointerstitial nephritis (TIN) in developed countries, following drug-induced cases, and the leading cause in developing countries. The infections leading to TIN can be categorized as follows:
- Bacteria: Escherichia coli, Campylobacter, Salmonella, Streptococci, Mycoplasma, Yersinia
- Viruses: HIV, cytomegalovirus, Epstein-Barr, polyomavirus, herpes simplex, COVID-19
- Fungi: Histoplasma, Coccidioides
- Parasites: Toxoplasma, Leishmania, Giardia
Patients with infection-related TIN usually present with symptoms of the underlying infection, and acute kidney injury (AKI) is rarely the initial symptom. Proper treatment of the infection is essential and should precede any steroid therapy used to manage TIN.
Join us to end the kidney disease epidemic
Autoimmune Tubulointerstitial Nephritis
Autoimmune diseases, including systemic lupus erythematosus (SLE), Sjogren’s syndrome, sarcoidosis, inflammatory bowel disease (IBD), and IgG4-related disease, are significant causes of tubulointerstitial nephritis (TIN).
- SLE: Lupus nephritis, affecting up to 40% of adults and 80% of children with SLE, traditionally involves glomerular injury, but recent evidence suggests interstitial fibrosis and tubular atrophy are better predictors of treatment response and prognosis. Despite advances, 10% of patients may progress to end-stage renal disease (ESRD).
- Sjogren’s Syndrome: Kidney involvement is rare (<10%) but typically manifests as TIN, characterized by plasma cell infiltration. Symptoms can include distal tubular acidosis and nephrogenic diabetes insipidus.
- Sarcoidosis: Granulomatous TIN is common in sarcoidosis and is often accompanied by altered vitamin D and calcium metabolism. Granulomas with epithelioid histiocytes are a key feature.
- IBD: Up to 23% of IBD patients may experience renal dysfunction, with TIN being the second most common cause. TNF-alpha may play a role in the inflammation of both IBD and TIN.
- IgG4-Related Disease: IgG4 disease causes tubulointerstitial damage, often presenting with proteinuria. This condition responds well to steroids but can mimic malignancy on imaging, so careful differential diagnosis is needed.
An Integrative Approach to Tubulointerstitial Nephritis
Given the diverse causes of tubulointerstitial nephritis, an integrative approach that combines conventional medical treatments with lifestyle and dietary changes can offer a more holistic way to manage the condition.
- Medication Review, Monitoring, and Deprescribing: Patients with TIN should work closely with their healthcare providers to review all medications and supplements they are taking. Monitoring kidney function regularly can help detect early signs of nephrotoxicity and allow for timely interventions.
- Nutrition and Hydration: Proper nutrition and hydration can worsen TIN. Patients with TIN may benefit from glutathione support by enhancing the intake of its precursor amino acids, such as cysteine, glycine, and N-acetyl cysteine. This can be achieved through a balanced diet that includes moderate amounts of sulfur-rich proteins—found in meats like beef, fish, and poultry—and a variety of sulfur-containing vegetables, particularly those from the allium family (such as garlic, onions, leeks, and chives) and cruciferous vegetables like broccoli and kale.Maintaining this balance is key and can be effectively managed with a predominantly plant-based diet that judiciously includes, rather than entirely excludes, animal proteins. This approach ensures adequate provision of essential nutrients while supporting kidney health. Staying well-hydrated can also support kidney function and help flush out toxins.
- Anti-inflammatory Therapies: Since inflammation is central to TIN, incorporating anti-inflammatory foods and supplements like omega-3 fatty acids (found in fish oil) may help reduce inflammation in the kidneys. Curcumin, a compound in turmeric, is another supplement known for its anti-inflammatory properties, though it should be used cautiously in people with kidney disease.
- Mind-Body Therapies: Stress can exacerbate inflammation and impact overall health, including kidney function. Practices like yoga, meditation, and mindfulness can help manage stress, reduce inflammation, and improve kidney health outcomes in patients with TIN.
- Herbal Medicine: While some herbs can be nephrotoxic, certain kidney-supportive herbs, such as astragalus and dandelion root, may be beneficial in promoting kidney health when used under medical supervision. However, it is crucial to consult with a healthcare professional before starting any herbal treatments to avoid adverse effects.
- Functional Medicine Testing: In integrative care, functional medicine testing can identify underlying imbalances, such as nutrient deficiencies, gut dysbiosis, or environmental toxin exposure, which may contribute to inflammation and kidney dysfunction. Tailoring treatment based on these insights can optimize outcomes for TIN patients.
The Bottom Line on Tubulointerstitial Nephritis
Tubulointerstitial nephritis is a complex condition with multiple causes, ranging from genetic factors and medications to supplements. While conventional treatments focus on managing symptoms and halting kidney damage, an integrative approach that addresses the root causes of inflammation and supports overall kidney health can enhance patient outcomes. Through careful medication management, a kidney-friendly diet, and the incorporation of stress reduction techniques, patients with TIN can take control of their health and work toward preserving kidney function for the long term.