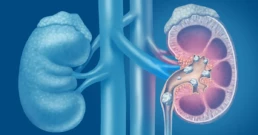
The Microbiome and Kidney Stone Formation
This blog is part of a series discussing our integrative approach to kidney stone prevention and management. In this blog we will focus on the microbiome and kidney stone formation.
Kidney stone formation (urolithiasis) is a complex disease influenced by multiple factors including diet, genetics, and environment. They are painful, inconvenient, and when left untreated, they may contribute to more serious conditions including obstruction and kidney damage.
Read more about the etiology and prevalence of kidney stones here.
In this series we’re building a case for a more integrative approach to preventing kidney stone formation.
Conventionally, the treatment approach does address kidney stones via a multi-pronged approach that may include medication, dietary and lifestyle, surgical removal, and using ultrasonic waves to break up stone.
However, these guidelines tend to focus too far downstream, on stone composition instead of on the underlying pathology upstream. Instead, we advocate for a more comprehensive approach that focuses on risk factors to prevent formation. Those factors include:
· Type of stone
· Socioeconomic factors
· Diet
· Hydration and electrolyte balance
· Microbiome and gut health
· Genetics
We covered individual dietary components in detail in a previous blog. Today we’ll look at the gut-kidney stone connection and the impact of the microbiome.
Gut Integrity and Kidney Stones: Leaky Gut
A normal and healthy GI tract has a natural barrier. This barrier serves to protect the GI and has three major jobs: 1. ensure proper digestion and absorption of nutrients and 2. ensure elimination of toxins and 3. protect the integrity of the microbiome – the “good” bacteria that lives in our GI tract and works with our body to maintain health.
Leaky gut describes a state when the cells that make up the lining of the GI tract separate enough to allow the contents of the gut to leak out. This is also sometimes called intestinal permeability or IP for short. This is a problem because it reduces absorption of nutrients, causes toxins to build up, alters the balance of the gut microbiome, and results in systemic inflammation.
One of the major contributors to leaky gut is the standard American diet (SAD), which seems to increase risk of kidney stone formation. When we use the term SAD, we are generally referring to a diet that includes:
· Consumption of sugary beverages and soda (and high carbohydrate consumption in general)
· Increased intake of processed/refined foods like cereals, crackers, baked goods, etc…
· Processed, fried, conventionally raised, high-nitrate animal protein
· Low intake of fiber and fresh produce in general
· A “beige” diet (low in phytonutrients and antioxidants) from consuming a variety of colorful fruits and vegetables
· Inadequate amounts of healthy, anti-inflammatory fats, and high amounts of refined unhealthy fats
We have already established that eating more fresh produce, is protective from kidney stone formation, and we’ve done a deeper dive on specific nutrition impact on kidney stone risk in another blog if you’d like to learn more.
There are several factors that may contribute to development of leaky gut:
· “Proinflammatory” SAD: too much processed and high-sugar foods, not enough fiber and the wrong inflammatory fats
· Food sensitivities: consuming food that are cause reactivity
· Overconsumption of caffeine and alcohol – irritants to gut lining
· Use of certain medications, including NSIADs, steroids, antibiotics
· Stress and poor-quality sleep
We address risk factors for intestinal permeability in more detail in a previous blog here, as well as dive into a comprehensive gut restoration strategy here in this 5-part series.
The Microbiome and Kidney Stones
Balance of the gut bacteria also play an important role in causing or preventing kidney stones. The most studied organism is Oxalobacter formigenes, which has been found to be protective when present in adequate quantities as part of the GI microflora. This bacterium degrades oxalate in the gut decreasing its absorption and excretion in the urine.
When Oxalobacter was discovered, scientists thought they had pinpointed the key to curing kidney stones. They concluded that simply supplementing this missing species should reduce risk of stone formation in susceptible individuals. It would turn out that the connection wasn’t that simple.
More recent evidence points to a more complex picture in the connection between microbiome diversity and kidney stone pathology. The emerging research shows increased risk in kidney stone formation in certain susceptible individuals also presented with alterations in normal microbiome and metabolome (metabolic byproducts from microflora) – also termed dysbiosis.
In other words, it’s likely that genetic factors might be “turned on” by dysbiosis leading to increased risk of kidney stone formation in certain individuals. The good news is that means they should be “turned off” when the microbiome balance is restored.
Studies that looked at the use of targeted probiotics have failed to show enough significant improvement of risk of urolithiasis. Although there’s been some limited and temporary reduction in oxalate excretion and kidney stone formation with the use of a combination of Lactobacillus, Bifidobacterium, Enterococcus, it’s been shown to be temporary and limited in benefit. This is because dysbiosis cannot be addressed by simply applying a band aid of a probiotic.
We recommend instead a more comprehensive approach to gut restoration and microbiome balance. You can read more about the 5R protocol in our comprehensive 5-part series on gut restoration.
The Bottom Line
Although initial findings about the impact of the microflora that looked at Oxalobacter in isolation have not demonstrated significance in reducing incidence of kidney stone formation, more recent evidence pointing to an interplay of factors on microbiome diversity is promising. Furthermore, factors that impact kidney stone formation include dietary factors, including food quality, nutrient composition, and dehydration. Along with environmental factors, lifestyle, genetics, and gut integrity and microbiome balance should be addressed through a comprehensive and personalized approach. Practitioners working with individuals to prevent kidney stone formation should formulate a patient care plan that modifies all relevant components in their integrative approach to maximize effectiveness in preventing urolithiasis.
Zinc and Kidney Disease, Exploring the Links
Zinc has been studied for years for its implications in kidney disease. In fact, many researchers have pointed to zinc deficiency as one of the underlying causes of the worsening of kidney disease. We can explore even more upstream and understand some of the factors that result in zinc deficiency in the first place. So let's discuss zinc and kidney disease.
Zinc: The Micromineral
Zinc is classified as a trace mineral because it’s typically needed in small doses to exert its biological effects. This nutritional mineral is involved in functions that include glycolysis (converting glucose into energy), digestion especially protein breakdown, bone formation, DNA metabolism, alcohol metabolism, angiotensin conversion, heme biosynthesis, and as an antioxidant protecting from reactive oxygen species (ROS) and the resulting cellular damage from inflammation. In fact, it’s so important, that it is the third most relevant intracellular ion (behind magnesium and potassium).
In addition, there are over 200 metal-containing enzymes, called metalloenzymes, responsible for catalyzing a variety of crucial reactions throughout the cells in our bodies. In fact, 20 distinct biological functions are associated with zinc metalloenzymes.
Zinc Absorption and Deficiency
Zinc is primarily absorbed in the small intestines via mechanisms called transcellular and paracellular transport. That means it can passively diffuse through tight junctions or by carrier mediated transporters. The latter means that absorption of zinc can be influenced by genetic SNPs which can result in decreased zinc transport and subsequently increased risk for deficiency.
Furthermore, absorption is reduced with aging and maldigestion/low stomach acidity. Drugs that inhibit stomach acid production, such as proton pump inhibitors (PPIs) and H2 antagonists (H2A) are thought to cause zinc depletion by reducing stomach acidity. This effect can be reversed by addressing the gut dysfunction through the 5R protocol.
Other drugs that may alter zinc status include angiotensin-converting enzyme inhibitors (ACEi), aspirin, thiazide and loop diuretics, long-term use of steroids, and fluoroquinolone, penicillamine, and tetracycline antibiotics. The degree of depletion is dependent on multiple factors, including individual differences, dose and length of therapy.
Assessment of zinc status through laboratory testing has been criticized as being unreliable. However, coupled with a comprehensive assessment of symptoms it can be very helpful in identifying mineral deficiency. Examples of symptoms of zinc deficiency include loss of/diminished taste and/or reduced sense of smell, frequent colds, slow wound healing, loss of appetite, presence of or worsening of acne/rosacea.
Zinc and kidney disease
The prevalence of zinc deficiency in chronic kidney disease (CKD) patients is well documented. Furthermore, zinc nutritional status is also altered in obesity. In fact, fasting plasma zinc concentrations are inversely correlated to BMI and plasma glucose levels.
Several inflammatory mechanisms are associated with zinc depletion, while adequate levels of zinc decreases formation of reactive oxygen species (ROS). ROS activate nuclear factor-κB (NF-κB) which induces the generation of inflammatory cytokines and adhesion molecules associated with various types of kidney diseases, including IgA nephropathy.
Superoxide dismutase (SOD), and enzyme which converts the oxidative superoxide into an inert product, hydrogen peroxide. The SOD enzyme requires zinc in order to work efficiently, and zinc deficiency reduces the activity of the enzyme and has been shown to increase inflammation-induced kidney damage, in part via this mechanism.
Another interesting associations include the impact of zinc deficiency on increased calcification in the development of kidney stones.
Zinc also plays an important role in protecting the kidneys from environmental toxins, especially heavy metal. Heavy metal toxicity, such as lead and cadmium, is significantly associated with kidney disease. Studies have shown that a deficiency in zinc, as well as other essential metals such as calcium or iron, can lead to increased absorption and toxicity of lead and cadmium. Therefore, providing yet another potential benefit of ensuring zinc repletion to protect against metal toxicity and its impact on kidney, as well as overall health.
Implications of zinc deficiency in cardiovascular and metabolic disease associated with KD
Zinc impacts kidney disease via indirect associations as well. Since cardiovascular disease (CVD) and diabetes are comorbid conditions associated with kidney disease, it makes sense that factors that impact them will also impact kidney disease.
It’s worth noting that various inflammatory markers that are triggered by deficiency of zinc, such as transcription of NF-κB, rise in IL-6, IL-2, and TNF-α, are associated with inflammatory/immune response that leads to systemic inflammation including atherosclerosis (vascular disease), a known complication of CKD.
Zinc has some interesting effects on blood lipids, including low density lipoproteins (LDL) and triglycerides (TG). Dyslipidemia is associated with increased risk of CVD. Zinc deficiency has also been associated with elevations in TG. Furthermore, maintaining adequate levels of zinc has a protective effect against atherosclerosis by inhibiting the oxidation of LDL by cells or transition metals.
In addition, there are multiple mechanism where zinc impacts metabolic disease. Interestingly, zinc is essential for insulin synthesis and release, and deficiency impairs the release of insulin contributing to insulin resistance and elevations in blood sugar.
Another hormone responsible for feeling of satiety (fullness after eating) is leptin, which is produced by fat cells. Leptin resistance is associated with overeating and obesity. Zinc depletion may impact leptin indirectly because leptin secretion is regulated by insulin. Furthermore, deficiencies in zinc impact the activity of peroxisome proliferator-activated receptors (PPARs). PPARs are associated with multiple metabolic pathways of lipid and glucose metabolism. They initiate cascade of mechanisms which activate atherogenesis, and are, therefore, central to metabolic disease, CVD, and kidney disease.
Food-based sources of zinc
Red meat, organ meats, and shellfish are considered the best sources of zinc, with levels significantly higher than vegetarian sources. Although not as high as animal sources, nuts and seeds are good sources of zinc. Although whole grains are good sources as well, refined grains are not because zinc is lost when the bran and germ coating is removed in processing. Unfortunately, although an essential part of a healthy diet, fruits and vegetables are not significant sources of this nutrient. Not surprisingly, vegetarians are at increased risk for zinc deficiency and should be screened routinely.
There are food interactions that reduce absorption of zinc. Phytic acid, naturally found in grains, may inhibit the absorption of zinc. Therefore it’s advisable to soak grains for 8-24 hours in filtered water and 1 tablespoon of lemon juice or apple cider vinegar prior to cooking to reduce levels of phytic acid and enhance absorption of zinc and other minerals. It’s also interesting to note that absorption of zinc is reduced by the intake of dairy, likely due to the high calcium content which is known to interfere with zinc uptake.
Supplementing zinc is a good option in cases of deficiency. There are multiple compounds available on the market. We recommend using zinc picolinate or zinc citrate. Zinc carnosine is useful as part of gut restoration protocol to address GERD and as part of a PPI tapering protocol. Note that the dosage and length of therapy should be customized to individual needs and tracked by your functional medicine provider or nutritionist. If you notice a metallic taste in your mouth, have your healthcare provider check your zinc levels to ensure they are not too high or toxic.
The Bottom Line
There are multiple suggested mechanisms contributing to the impact of zinc deficiency on kidney health. It’s no surprise considering the various mechanisms that involve zinc. It’s important to consider the impact of diet, medications, dysbiosis and intestinal permeability on zinc status and subsequently on protecting the kidneys from disease.
The 5R Protocol Part 5: Rebalance
This is part of a series of blogs discussing an individualized comprehensive gut restoration protocol in chronic kidney disease. Here, we talk about the final step: Gut rebalance and kidney health.
The Gut-Kidney Connection
Recent studies have focused on the significance of a relationship between gastrointestinal (GI) integrity and microbiome diversity with various chronic diseases including kidney disease.
In previous blogs, we discussed the impact of exposure to food and environmental triggers that impact the gut lining (or mucosa)integrity and microbiome balance leading to intestinal permeability (IP or “leaky gut”). The impact of leaky gut on kidney health and progression of chronic kidney disease (CKD) has been referred to as the gut-kidney connection is the result of complex biochemical and immune mechanisms.

A comprehensive approach to CKD includes addressing the health of the GI at the root of it. Furthermore, this means not simply supplementing with probiotics, but instead addressing all the mechanisms that underlie probiotic need. This includes modification of microbiota balance, integrity of the mucosa and epithelium of the GI tract, improving GI motility, absorption and digestion, and modulation of the immune system.
Below we will explore the fifth step, Rebalance. But first, let’s quickly review the first four steps of the comprehensive gut restoration protocol. A reminder that the 5R Protocol addresses leaky gut as a foundational approach to reduce the risk of progression of CKD.
The 5 steps of the 5R protocol for healing leaky gut are:
1) Remove potential triggers, including polypharmacy, pathogenic organisms, food intolerances, sensitivities and allergies, or toxic exposure.
2) Replace digestive aid to support improved nutrient absorption and metabolism, including digestive enzymes, or agents that promote improved motility and regular bowel movements.
3) Reinoculate provide an environment where good bacteria can thrive and where bad ones cannot.
4) Repair support of the cellular repair process through the above, as well as by providing specific nutritional support for the regeneration of the GI protective barrier.
5) Rebalance lifestyle factors that influence the gut bacteria such as stress, sleep, exercise and relationships and assure ongoing gut health.
Rebalance
The goal of the fifth step is maintenance and prevention of recurrence of IP or leaky gut. There are various factors that lead to IP that involve dietary and lifestyle influences*, including:
· Standard American Diet (SAD) which is low in fiber, high in processed foods, and highly inflammatory
· Poor eating habits (for example, multitasking and not chewing adequately)
· Inadequate hydration and/or electrolyte imbalance
· Motility issues leading to constipation or unfavorable formation/frequency of stool
· Stress and poor sleep
· Not enough exercise
To reduce risk of CKD, we must work towards improving diet and lifestyle habits that support continued GI health.
*Read more about medications that impact gut health and ultimately increase risk for KD progression here.
Where to start?
Address lifestyle factors that impact gut health
Long term dietary goals focus on a plant-based diet that is high in fiber and a wide range of key nutrients. Organic sources of animal protein can beneficial when eaten in moderation. However, the key is to load up on naturally antiinflammatory, low carbohydrate vegetables to maximize vitamins, minerals, phytonutrients and, of course, fiber. Reduce intake of starchy vegetables and eat more**:
· Dark leafy greens (like spinach, arugula, and romaine)
· Cruciferous veggies (like broccoli, cauliflower, and kale)
· Fresh whole fruit (preferably lower sugar berries and avocados, and limit to two servings per day)
· Some colorful starchy veggies can be OK (for example, carrots, sweet potatoes, beets, and squashes)
Those veggies listed above promote a healthy microbiome and improve alkalinity associated with improved kidney health. In addition to those sources of prebiotic fiber, include probiotic sources like fermented vegetables and drinks like raw sauerkraut, kimchi, pickles, apple cider vinegar, and kombucha. It’s recommended these are eaten raw because pasteurization process will destroy the bacterial content of these foods.
In general, reduce processed carbs, like bread, cereals, and high sugar foods, desserts and pastries. These types of foods are usually low in fiber and nutrients and help contribute to starving of beneficial gut bacteria. Furthermore,high carb diets have been associated with increased risk of cardiovascular (CV) disease and diabetes. Instead, moderately consume whole grains like brown rice, quinoa, oatmeal, and legumes**.
Include antiinflammatory fats focusing primarily on omega 3 sources from fish, nuts, seeds, and mono- and polyunsaturated (MUFA and PUFA) like avocados and olive oil. Even though the topic of saturated fats is more controversial, recent evidence suggest that moderate intake of certain saturated fats like that found in beef, organ meat, or ghee (clarified butter) derived from grass-fed cows and virgin coconut oil might have health benefits. Everyone agrees, however, that trans fats(aka hydrogenated oils and artificial products like margarine) or excessive intake of processed and fried fats contributes to inflammation and increased risk of disease.
Ensuring adequate hydration, drinking at least half your body weight (pounds) in ounces of water, not only helps to maintain good kidney health, it also helps support daily regular bowel movements.
It’s well established that regular exercise can be beneficial for many reasons, including improved blood pressure, blood sugar, hypertension, stress relief, and even improved digestion and GI motility. In fact, exercise has been associated with improved microbiome balance as well as beneficial modulation of the immune system.
Poor sleep quality and stress are also deeply tied to many underlying factors impacting of GI health. Reduced sleep duration and quality has been associated with increased inflammatory markers (including TNF, IL-1, and IL-6) associated with GI disease like GERD and Irritable Bowel Disease/Syndrome (IBD/IBS) disrupting digestion and nutrient absorption. Furthermore, sleep has been shown to affect kidney health directly and indirectly, including associated risk of CV disease, diabetes, obesity, and hypertension(read more about the relationship between sleep and kidney health here).
**NOTE: Due to individual variations and progress of disease, work with a nutritionist to assess if you need to maintain any specific restrictions due to your unique case and needs.
The role of supplements
Because of the unique needs of kidney disease patients, many need to rely on supplements to help obtain adequate amounts of key nutrients to maintain GI and kidney health.
This may include GI and motility support including but not limited to digestive enzymes, bitters, probiotics, and magnesium citrate and triphala for motility). Furthermore certain individuals may benefit from supplementation of certain vitamins and important minerals, high potency antioxidants, and/or support of certain key underlying cellular mechanisms impacting mitochondrial health, detoxification and nitric oxide production.
That said, many factors must be taken into consideration when choosing appropriate supplements for each patient. Supplement quality and contamination are a common concern, as are potential interactions with medication or contraindications in certain commonly associated chronic disease. We suggest working under the care and guidance of a practitioner or team of providers who are trained in integrative and functional medicine and understand the unique needs of kidney patients.
Next steps
Unfortunately, addressing gut health is only the beginning. As mentioned above, kidney disease is often associated with multiple chronic diseases including CV disease, diabetes, high blood pressure, and obesity.
The comprehensive approach to kidney care means addressing the underlying causes of this constellation of diseases which is best accomplished by a Functional Medicine approach. The goal is to identify and then rebalance the biochemical and pathophysiological dysfunction at the root of chronic disease, we can stop the progression of kidney damage and preserve kidney function.
Bottom Line
In the final step in the 5R individualized gut restoration protocol, we Rebalance the foundational factors that impact the gut-kidney axis.
Although this might be the last step in the 5R protocol, within the broader context of kidney disease, it might signal the transition to a comprehensive therapeutic protocol that includes management of the underlying dysfunction associated with related conditions such as CV disease, diabetes, obesity, and hypertension.
Working with an integrative or functional medicine provider is essential to help you navigate the comprehensive program successfully, but can help you stabilize blood sugar, lower blood pressure, lose weight, and reduce the risk of CV and ultimately, KD.
More from InKidney on the gut-kidney connection:
· Comprehensive Gut Restoration Protocol https://inkidney.com/2019/07/05/comprehensive-gut-restoration-protocol-ckd/
· Feeding Gut Bacteria in Kidney Disease https://inkidney.com/2019/02/20/feeding-gut-bacteria-patients-kidney-disease/
· Kidney-Gut Axis: Nutrition can slow the progress of kidney disease https://inkidney.com/2018/09/20/kidney-gut-axis-nutrition-slow-kidney-disease/
· Inflammation, Leaky Gut And Kidney Disease https://inkidney.com/2018/10/05/inflammation-leaky-gut-kidney-disease/
· Leaky Gut And Kidney Disease: 6 Classes Of Medication That Might Be Contributing https://inkidney.com/2018/10/05/leaky-gut-kidney-disease-medications/
The 5R Protocol Part 4: Gut Repair and Kidney Health
This is part of a series of blogs discussing an individualized comprehensive gut restoration protocol in chronic kidney disease. In this blog, we will talk about gut repair and kidney health.
The Gut-Kidney Connection
The gut-kidney axis refers to the relationship between gut integrity and microbiome diversity with kidney disease. Excessive intestinal permeability, also known as hyperpermeability or more commonly as “leaky gut,” has been shown to be at the root of this connection. This gut-kidney relationship is the result of complex biochemical and immune mechanisms.
So far we looked at the first three steps of the 5R protocol, Remove , Replace, and Reinoculate. Applied sometimes sequentially and at times simultaneously, these steps are used to address the underlying factors associated with leaky gut. The idea is that comprehensive approach that reveres damage to the gut caused by exposure to food and environmental triggers, addresses the disruption of digestion and nutrient absorption, altered bowel motility, and dysbiosis, improves gut health and ultimately overall kidney health.
Read on below where we will explore step 4, Repair. But first, let’s first review the five steps of the comprehensive gut restoration protocol. A reminder that the 5R Protocol addresses leaky gut as a foundational approach to reduce the risk of progression of CKD and in our upcoming blog on Rebalance, we will explore maintenance and next step in integrative kidney care.
The 5 steps of healing leaky gut are:
1) Remove potential triggers, including polypharmacy, pathogenic organisms, food intolerances, sensitivities and allergies, or toxic exposure.
2) Replace digestive aid to support improved nutrient absorption and metabolism, including digestive enzymes, or agents that promote improved motility and regular bowel movements.
3) Reinoculate provide an environment where good bacteria can thrive and where bad ones cannot.
4) Repair support of the cellular repair process through the above, as well as by providing specific nutritional support for the regeneration of the GI protective barrier.
5) Rebalance lifestyle factors that influence the gut bacteria such as stress, sleep, exercise and relationships and assure ongoing gut health.
Gut Repair and Kidney Health
Up until this phase, we’ve focused on removing triggers that contribute to local and systemic inflammation. We’ve even taken steps to rebalance the microbiome. In the repair phase, we work to provide nutritional support that directly impacts the integrity of the gut mucosa and repairs hyperpermeability.
Recall from our previous blog, Part 1: Remove, that exposure to toxins, food sensitivity, and presence of pathogens leads to increased inflammation locally that triggers the immune system and leads to damage to the lining of the gut and mucosa.
The term Intestinal hyperpermeability (aka “leaky gut”) is the result of this inflammatory assault to the gut lining. These breaks in the integrity of the wall (imagine gaps in a fence on your lawn) let undigested food, bacteria, and metabolites “leak” through the holes.
The physiologic changes associated with this leaky state include a combination of factors that reduce your gut’s ability to absorb nutrients. These include hypochlorhydria (insufficient hydrochloric acid in the stomach to digest food), reduced production of digestive enzymes, altered bowel motility (often leading to constipation, but not always), and dysbiosis.
Now that we’ve addressed those aspects in steps 1-3, we can focus our energy on repair. This stage is often several weeks into the comprehensive gut restoration program because initiating it simultaneously may interfere with the efficacy of the prior steps. Working with an integrative or functional medicine practitioner can help guide you through your personalized program.
Where to start?
Address permeability with nutrients and herbs
To support intestinal mucosa regrowth and cell repair we focus on nutrients that have properties that promote rebuilding a healthy mucosal lining. They help improve the integrity of the intestinal wall by supporting building and formation of the intestinal epithelium, villi and cell connective tissue.
This includes a wide spectrum of micronutrients including vitamin A, D, E, and C. L-glutamine, butyric acid, and collagen supplements. These are useful to build collagen that forms the epithelium of the GI tract. Whey, colostrum or serum bovine immunoglobulins may be utilized to balance inflammatory mediators based in the gut. Zinc carnosine, melatonin, cabbage juice, aloe vera, and mucilaginous herbs like marshmallow root and slippery elm are also used therapeutically to support various aspects of the rebuilding process.
This is of course layered in on top of an anti-inflammatory, nutrient-dense, fiber-rich diet that includes healthy fats, moderate intake of animal protein, and a colorful variety of organic fruits and vegetables that provide added antioxidant and phytonutrient repair support.
The Bottom Line on Gut Repair and Kidney Health
The fourth step in an individualized comprehensive gut restoration protocol involves leveraging food and herbs to promote the repair of the gut mucosa. This is often done after Remove, but might be simultaneous to Replace and Repair steps. However, every case is unique, and it’s important to work with an integrative or functional medicine provider trained in the comprehensive gut restoration protocol to help you navigate this safely and successfully. Next, we will tackle the 5th “R” in the gut restoration protocol: Rebalance.
Dietary Approach to Kidney Stone Prevention
This blog is part of a series discussing our integrative approach to kidney stone prevention and management. In this blog we will discuss the dietary approach to kidney stone prevention.
Kidney stone formation (also called urolithiasis or nephrolithiasis) is a complex disease influenced by multiple factors including diet, genetics, and environment. Anyone who’s experienced them can attest that stones are often very painful. When left untreated, they may contribute to more serious conditions including urinary tract obstruction and permanent kidney damage or CKD.
Read more about the etiology and prevalence of kidney stones here.
By Lara Zakari, PharmD, CNS, CDN, IFMCP
Conventionally, the approach to treatment is a multi-pronged approach and may include medication, dietary and lifestyle interventions, surgical removal, and using ultrasonic waves to break up stone. However, dietary guidelines tend to focus on stone composition instead of on the underlying pathology. In this series, we’ve been discussing the combination of upstream risk factors that impact risk of stone formation.
Factors that impact the integrative approach to kidney stones include:
- Type of stone
- Environment
- Diet
- Hydration and electrolyte balance
- Microbiome and gut health
- Genetics
Diet is a topic that deserves a deeper dive! We’ll focus on it here today.
Dietary Approach to Kidney Stone Prevention
The Diet Controversy and Kidney Stones
Dietary interventions are common in addressing kidney stones. The reality is, we need a more comprehensive approach when it comes to preventing kidney stone formation and kidney damage. Dietary approach to kidney stone prevention is one aspect of this comprehensive integrative approach.
First, let’s start by outlining the impact of the standard American diet (SAD), which seems to increase risk of kidney stone formation. When we use the term SAD, we are generally referring to a diet that includes:
- Consumption of sugary beverages and soda (and high carbohydrate consumption in general)
- Increased intake of processed/refined foods like cereals, crackers, baked goods, etc.
- Processed, fried, conventionally raised, high-nitrate animal protein
- Low intake of fiber and fresh produce in general
- A “beige” diet (low in phytonutrients and antioxidants) lacking color and low in enough variety of colorful fruits and vegetables
- Inadequate amounts of healthy, anti-inflammatory fats, and high amounts of refined unhealthy fats
Interestingly, studies have shown that eating more fresh produce is protective from kidney stone formation. When we consider that the SAD is low in protective foods and the key nutrients found to be helpful in preventing urolithiasis. Let’s examine these factors that impact kidney stones in more detail.
Macronutrient Balance for Kidney Stones (Protein, Fat & Carbs)
Protein
For a long time, it was assumed that stone formation was at least in large part due to excessive protein intake. As a result, those at risk for stone formation were instructed to eat a low protein diet to prevent urolithiasis. However, there are underlying factors that cause a domino effect impacting how the products of protein breakdown lead to stone formation. This includes micronutrient and electrolyte balance, metabolic disorders, digestive abnormalities, and of course dysbiosis (we’ll discuss these in detail in another blog).
It’s true that excessive protein intake can lead to more acidic urine and increased uric acid production (a risk factor for uric stone formation). Elevated protein metabolism alone isn’t a problem in isolation, there’re other upstream factors to consider that make certain individuals more susceptible to stone formation. Furthermore, the source of protein seems to be significant. Animal protein sources seem to play a bigger significance in risk of stone recurrence, while plant-proteins might be protective.
Carbohydrates and Fiber
Though excessive consumption of carbohydrates isn’t recommended, excessive restriction of carbs in people prone to kidney stones should be avoided. We suggest avoiding sources of simple sugars, fructose and high fructose corn syrup (that includes sugar sweetened beverages, pastries, sodas, and even the so-called healthy sweetener agave). Fructose consumption in particular seems to increase production of excess uric acid and simultaneously reduces its excretion in the urine.
Instead, we want to opt for more nutrient-dense, high-fiber, low-glycemic carbohydrates, including root vegetables, berries, whole grains and legumes. Increased consumption of fiber helps to promote improved microbiome health and diversity, which may play a significant role in kidney stone formation risk. Furthermore, there is interesting research that indicates that individuals who aren’t getting enough dietary vitamin C to meet their needs while on a low-carb diet may increase their risk for developing certain types of kidney stones.
This begs the question, what other nutrient deficiencies might contribute to stone formation when macronutrients might not be optimized?
Fats and Essential Fatty Acids
In order to balance a moderate protein and carb intake, some individuals might benefit from increasing calories from fat. Healthy fats include omega 3 sources like fish and fish oil, nuts, seeds, olive oil, and avocado. Grass-fed ghee might be especially beneficial due to a combination of anti-inflammatory profile, vitamin A, and gut healing benefits of the butyric acid component.
Independently, increased fat intake has not been associated with kidney stone formation. However, when coupled with a SAD diet full of simple carbohydrates and fried and processed foods, and excessive protein intake that seems, at least in part, to be a contributing factor. At the same time, when eating a high-fat diet as part of a ketogenic approach, excessive restriction of carbohydrates seems to contribute to stone formation in individuals with existing risk factors. In those cases, it’s recommended to work with a nutritionist to ensure that you’re balancing your macronutrients effectively to avoid kidney stones.
Micronutrient Balance
Fat-soluble vitamins
We can't talk about dietary approach to kidney stone prevention without discussion fat soluble vitamins. Fat soluble vitamins include vitamins A, D, E, and K. Deficiencies in D, A and K in particular have been associated with kidney stone formation. This might at least in part be due to the fact that these vitamins play a major role in calcium metabolism and bone mineralization. When D and K are deficient, calcium from diet is deposited in arteries instead of in bones, leading to calcification and stone formation.
In fact, vitamin K depletion might be an independent risk factor for kidney stone formation. When individuals form kidney stones, a vitamin-K dependent protein (matrix Gla protein) is secreted in inactive form. This protein when activated with vitamin K can inhibit the growth of calcium oxalate crystals. When vitamin K isn’t available, more crystals may form.
To improve dietary intake of these nutrients, foods like eggs (especially the yolks), organ meats, ghee, natto (fermented soybeans), and full-fat cheeses (assuming you’re able to tolerate dairy) can be helpful to include in your diet. Daily sun exposure (at least 20 minutes a day) can also help promote increased vitamin D levels. However, supplementation is often indicated. Issues with absorbing fats and fat-soluble vitamins due to digestive issues are common, a nutritionist or a clinician trained in Integrative and Functional Medicine can help you determine if supplementation is needed.
Oxalates
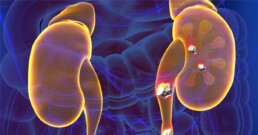
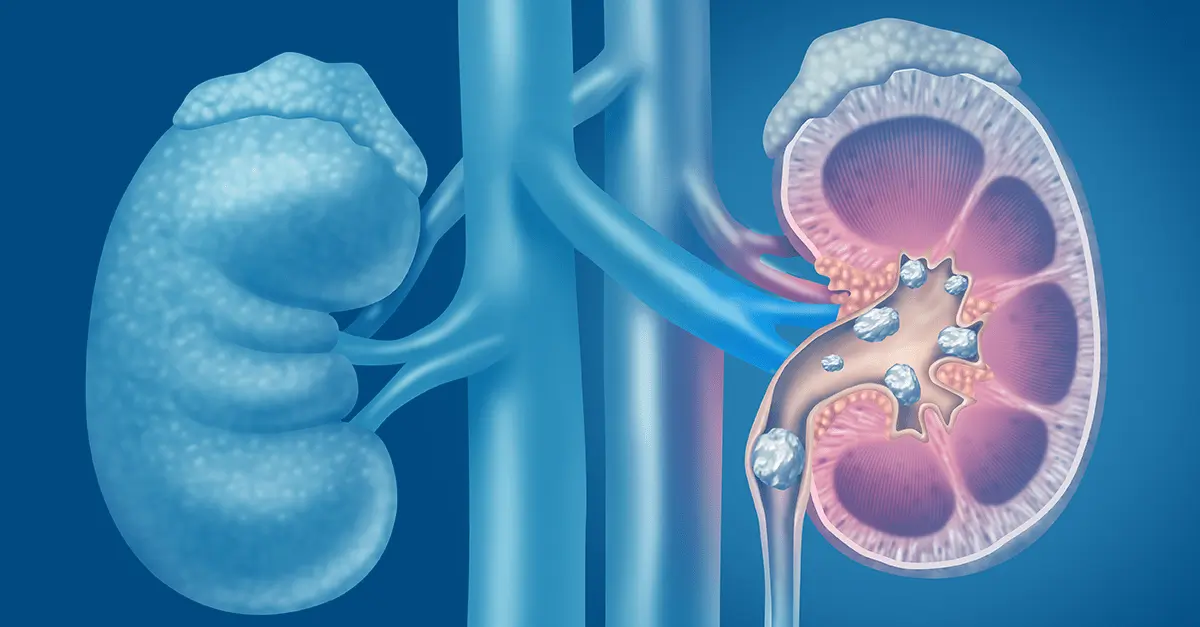
Oxalates are front and center in the dietary approach to kidney stone. In fact, calcium oxalate stones comprise most kidney stones (75%). There’s some evidence that limiting consumption of oxalates can help reduce stone formation. Food containing high levels of oxalates tend to be generally considered healthy, including spinach, beets, nuts and seeds, to name a few examples.
What makes these foods healthy for some people, and contribute to stone formation in others?
One reason might be inadequate calcium intake from the diet. Food sources like broccoli, sardines and canned salmon, and leafy greens like kale and collard greens can be good low-oxalate, calcium-rich food sources. When consumed in adequate amounts, calcium binds to oxalates in the intestines, preventing it from being absorbed and reducing formation of stones.
Another important consideration is the composition of the microbiome. When oxalate-degrading bacteria in the gut is inadequate, it can increase risk of developing kidney stone disease. More about that in a future article.
Vitamin C
Ascorbic acid has been associated with formation of kidney stones. Excessive intake of vitamin C might increase oxalate production. Some studies have shown higher intake of vitamin C is associated with increased risk of kidney stones, but only in people predisposed to forming oxalate-stones. Regardless, caution should be used in supplementing vitamin C within the context of the larger, integrative picture.
Phytate
Phytate, sometimes called phytic acid, is an antioxidant compound found in certain foods like beans, lentils, nuts, and seeds. Some have claim that phytate intake might prevent the absorption of certain nutrients, but actually it might be protective for kidney stone! One study found that women who consumed more phytate had reduced risk of kidney stone formation. Other studies have come to the same conclusion. This might account to the benefit of eating these particular foods, which happen to be good plant-based protein sources, in place of animal protein.
Salt and Other Electrolytes
Calcium is a controversial topic when it comes to risk of kidney stone formation. Surprisingly, higher intake of dietary calcium (from food sources like leafy greens, salmon, and legumes, for example) is associated with lower risk of stone formation. On the other hand, taking calcium supplements may be linked with increasing risk of kidney stone formation (this might be gender specific to men).
Furthermore, the conversation often fails to address the nuance of calcium consumption in the form of dairy, which might not be superior over plant-based forms of calcium, or to account for the impact of vitamin D deficiency.
Magnesium is another electrolyte that is often overlooked. Magnesium is part of hundreds of the body’s biochemical reactions, so adequate intake is essential. Furthermore, ensuring adequate intake of magnesium can help prevent the formation of calcium oxalate stones by destabilizing the bond between the two compounds causing them to split.
Food sources of magnesium include swiss chard, spinach, almonds, and pumpkin seeds. Many of those foods might be contraindicated in a low-oxalate diet, so supplementation might be indicated before gradually adding those foods back as part of a comprehensive approach. Supplementing with chelated forms of magnesium (like magnesium citrate, malate, or bisglycinate) or making time for an epsom salt bath (magnesium sulfate in the salts absorbs through the skin).
But the most important electrolyte in the dietary approach to kidney stone is sodium. Excessive sodium (salt) consumption has been associated with stone formation, particularly when fluid intake is inadequate. High sodium excretion increases calcium deficiency by increasing excretion through the urine. Reducing excessive intake of sodium, especially from processed foods like cured meats, can be helpful, along with ensuring adequate intake of potassium-rich foods like broccoli, leafy greens, squash, potatoes, mushrooms, bananas, cantaloupe, and grapefruit to name a few.
More about the impact of electrolyte balance on kidney stone formation in a future blog.
Water (the forgotten macronutrients)
Inadequate water intake is one of the most significant contributing factors to kidney stone formation. The process of excretion of oxalate consumes salt and water, as a result dehydration is more likely.
Taking mineral compounds containing citrate (like magnesium citrate or potassium citrate) can prevent the formation calcium oxalate and uric acid stones while balancing mineral needs. Adding lemon juice or apple cider vinegar (4oz per day) to water might also help prevent urolithiasis through the same mechanism. Your Integrative or Functional Medicine clinician can help create a plan that best fits your needs.
The Bottom Line
Multiple factors impact kidney stone formation, including dietary factors. It seems there’s no single food or food component that causes stone formation. Rather, there are likely a combination of factors including nutrient depletion, dehydration, and poor food choices combined with environmental factors, genetics, and gut integrity and microbiome balance which combined play a role.
Therefore, the integrative approach to addressing kidney stones must account for the constellation of these factors. Practitioners working with individuals to prevent kidney stone formation should formulate a personalized approach that modifies all relevant components in their integrative approach. We’ll explore these connections in future blogs in this series.
The 5R Protocol Part 3: Reinoculate
This is part of a series of blogs discussing an individualized comprehensive gut restoration protocol in chronic kidney disease.
The Gut-Kidney Connection
In this series we examine the comprehensive gut restoration protocol used to as a foundational approach to heal gut integrity. Researchers have established a relationship between gut integrity and microbiome diversity with various chronic diseases, including kidney disease. Increased intestinal permeability, also known colloquially as “leaky gut” has been shown to be at the root of this connection. This gut-kidney relationship is the result of complex biochemical and immune mechanisms.
In our previous blogs, we discussed the impact of exposure to food and environmental triggers that impact the gut lining (or mucosa)integrity and microbiome balance leading to intestinal permeability (aka “leaky gut”).
So far we have looked at how the first and second steps of the 5R protocol, Remove and Replace, help to address the underlying factors associated with leaky gut that include a combination of factors like exposure to food and environmental triggers causing local and systemic inflammation, disruption of digestion and nutrient absorption, altered bowel motility, and dysbiosis.
Below, we will explore step 3, Reinoculate. Let’s first review the five steps of the comprehensive gut restoration protocol. The 5R Protocol addresses leaky gut as a foundational approach to reduce the risk of progression of CKD. The five areas of GI mucosal integrity are:
1) Remove potential triggers, including polypharmacy, pathogenic organisms, food intolerances, sensitivities and allergies, or toxic exposure.
2) Replace digestive aid to support improved nutrient absorption and metabolism, including digestive enzymes, or agents that promote improved motility and regular bowel movements.
3) Reinoculate provide an environment where good bacteria can thrive and where bad ones cannot.
4) Repair support of the cellular repair process through the above, as well as by providing specific nutritional support for the regeneration of the GI protective barrier.
5) Rebalance lifestyle factors that influence the gut bacteria such as stress, sleep, exercise and relationships and assure ongoing gut health.
Reinoculate
The basic premise of the third stage of gut restoration is to foster an environment that allows beneficial microflora (aka good bacteria) to thrive in the gastrointestinal (GI) tract. This is achieved by leveraging diet, supplementation, and lifestyle modification.
In previous blogs, we have explored the connection between dysbiosis and kidney health. An increasing number of studies have demonstrated a significant relationship between the health of the microbiome and the progression of kidney disease, a relationship referred to as gut-kidney axis.
Those with a healthy microbiome, abundance of good bacteria and no overgrowth of bad bacteria, are less likely to develop chronic kidney disease. In fact, the presence of certain strains of bacteria in the gut can actually slow the progression of chronic kidney disease and even reduce the need for dialysis.
Where to start?
Prebiotics (aka Fiber)
Most Americans fall significantly short of the recommended fiber intake of 30+ g/day. High fiber intake is associated with reducing the risk of heart disease, obesity, diabetes, and even certain kinds of cancers like colorectal cancer. These benefits are linked to to improved bowel movements, neutralizing and removing toxins, and “feeding” gut bacteria contributing to a favorable microbiome balance.
Research even suggests that dietary fiber-intake may be one of the most significant predictors not only of gut health, but overall health and risk of disease!
Remember to always choose whole, fresh, fiber-rich fruits and veggies whenever possible to maximize nutrients and prebiotics simultaneously. Whole grains and legumes make good fiber sources as well, but always avoid processed foods that claim to have “added fiber”. They are usually packed full of fillers, sugars, grains, cereals and artificial ingredients.
High-sugar diets can be a major disrupter of your gut microbiome, primarily because it feeds bad bacteria and yeast overgrowth. This is one of the proposed mechanisms contributing to metabolic diseases like diabetes and heart disease, two conditions associated with KD.
Prebiotic supplementation might also be necessary in some cases. Supplemental powders and capsules of resistant starch, arabinogalactan, and mastic gum, among others, might be useful in many situations. Your integrative or functional medicine provider can help you determine which, if any, are appropriate for your unique situation.
Probiotics
Supplementation with high intensity probiotics may be very useful in the Reinoculation phase of the 5R protocol. There are a variety of strains of bacteria and even beneficial yeast that are used to help to “seed” the gut (though technically more recent studies suggest the benefit is transient, it can still be helpful). Depending on your individual needs, your integrative and functional medicine provider may choose a particular strain or opt for a broad-spectrum formulation. Either way, the quality and potency of the probiotic is important to consider (not all brands are created equal).
That said, research seems to support that the best long-term strategy is to increase the intake of probiotics coupled with prebiotic fiber intake through diet. Traditionally fermented foods are a great source of naturally found probiotics, these include non-pasteurized traditionally made kefir, sauerkraut, kimchi, miso, and pickled vegetables.
Other factors impacting the microbiome
Interestingly, exercise impacts microbiome balance and promote changes that improve gut health. Aim for at least 20 minutes of exercise daily at a level appropriate to your physical fitness and make it priority to get up and move - you and your kidneys are worth it!
Furthermore, stress has been found to negatively alter the balance of the microbiome by reducing the presence of friendly flora and promote the growth of bad bacteria. Although we know that stress cannot always be avoided, everyone can find a stress management practice to reduce its negative impact. Breathing exercises, meditation, long walks, listening to music, adult coloring books – the options are endless. Find what works best for you!
Bottom Line
The third step in an individualized comprehensive gut restoration protocol involves promoting microbiome balance. This is often done after the Remove step but might be simultaneous to the Replace step. Sequencing the steps of the protocol is case-by-case dependent. It’s important to work with an integrative or functional medicine provider trained in the comprehensive gut restoration protocol to help you navigate this successfully.
Next, we will tackle the fourth “R” in the gut restoration protocol: Repair.
The 5R Protocol Part 2: Replace
This is part of a series of blogs discussing an individualized comprehensive gut restoration protocol in chronic kidney disease.
The Gut-Kidney Connection
Researchers have established a relationship between gut integrity and microbiome diversity with various chronic diseases, including kidney disease. Increased intestinal permeability, also known colloquially as “leaky gut” has been shown to be at the root of this connection. This gut-kidney relationship is the result of complex biochemical and immune mechanisms.
In our previous blog, we discussed the impact of exposure to food and environmental triggers that impact the gut lining (or mucosa)integrity and microbiome balance. Intestinal permeability (aka “leaky gut”) is the result of inflammatory assault to the gut lining that results in breaks in the integrity of the wall. Imagine it like breaks in a “fence” that let undigested food, bacteria, and metabolites “leak” through the holes.
The physiologic changes associated with this leaky state include a combination of factors including hypochlorhydria (insufficient stomach acid to break down food adequately), reduced production of digestive enzymes, altered bowel motility (often leading to constipation, but not always), and dysbiosis.
As a result of these pathologies, leaky gut leads to increased local and systemic inflammation, poor digestion, reduced nutrient absorption, and reduced ability to remove toxins and digestive byproducts.
Though we address step two, Replace in more detail below, let’s review the five steps of the comprehensive gut restoration protocol. The 5R Protocol addresses leaky gut as a foundational approach to reduce the risk of progression of CKD. The five areas of GI mucosal integrity:
1) Remove potential triggers, including polypharmacy, pathogenic organisms, food intolerances, sensitivities and allergies, or toxic exposure.
2) Replace digestive aid to support improved nutrient absorption and metabolism, including digestive enzymes, or agents that promote improved motility and regular bowel movements.
3) Reinoculate to provide an environment where good bacteria can thrive and where bad ones cannot.
4) Repair support of the cellular repair process through the above, as well as by providing specific nutritional support for the regeneration of the GI protective barrier.
5) Rebalance lifestyle factors that influence the gut bacteria such as stress, sleep, exercise and relationships and assure ongoing gut health.
Replace
Due to diet quality, food sensitivities, pathogenic microbes, epithelial inflammation leads to damage to the gut mucosa integrity. This causes damage to the structure of the villi and reduction in the intestinal surface area leading to reduced absorption of nutrients. This is referred to as malabsorption, or poor absorption of nutrients across the gut wall into circulation to be used by the body.
A second pathology is maldigestion, or ineffective breakdown of nutrients from food. This might be one or more of the major macronutrients, fat, protein, and/or carbohydrates. In these cases, you’ll notice GI distress after eating, bloating, distention, and or excessive flatulence. This not only reduces the effective absorption of essential nutrients necessary for healthy metabolism, but also creates a backup that contributes to increased inflammation, buildup of endotoxins, and further contributing to dysbiosis.
Furthermore, poor motility can contribute to chronic constipation. It’s been estimated that 20% of the adult population suffers from chronic constipation – that’s 1 in 5 adults! Ideally bowel movements should be daily, easy to pass and as characterized by the Bristol Stool Scale below, smooth soft and formed.
Constipation is not only uncomfortable, but leads to inefficient removal of toxins, recirculation of endogenous hormones, and alteration of the microbiome balance. Furthermore, stool consistency and frequency can be used as clues for clinicians signaling the need for a gut restoration protocol. From there, integrative and functionally trained practitioners can use advanced stool testing that employs a technique called PCR technology as discussed in Part 1 Remove blog.
Where to start?
Assess Nutrient status
Often, we begin by assessing the baseline nutrition status for any micro- or macronutrient deficiencies. These tend to be more significant with long-term maldigestion and malabsorption, depriving the body of necessary vitamins, minerals, amino acids, and essential fats needed for healthy function. Supplementation is often necessary, but dietary adjustments can be made to optimize absorption from food as long as digestion and motility are also addressed (see below).
Assessment can be done by physical exam to detect signs and symptoms of nutrient deficiency – for example dry hair and skin, brittle nails, bleeding gums, and difficulty digesting animal protein or fat can all be indications of various nutrient malabsorption/depletion or maldigestion.
Furthermore, laboratory assessment of nutrient status can confirm specific deficiency and help us target key nutrients strategically to customize the therapeutic approach.
Address Maldigestion and Malabsorption
To enhance digestion, use of broad-spectrum digestive enzymes such as lipase and bile salts, proteases, and cellulases that break down fats, proteins and carbohydrates, respectively is necessary because the digestive tract isn’t producing sufficient enzymes to breakdown and absorb nutrients form food. In addition, betaine HCL, a digestive aid that address low hydrochloric acid (hypochlorhydria) in the stomach may also be necessary to improve gastric pH.
Herbs and foods can also be useful for improving nutrient absorption and motility. For example, pineapple, papaya, and bitter foods like dandelion greens can be helpful for aiding digestion, stimulating motility and digestive enzyme production. Eating beets and staying hydrated promotes bile salt flow. Fermented and fiber-rich foods can also help promote motility and healthy microbial balance (though sometimes we don’t introduce these until step 3: Reinoculate). Last but not least, traditional concoctions of herbal bitters and teas can be very helpful in promoting this process.
Address Motility
We cannot emphasize enough how important it is to have at least one daily bowel movement for overall health. That is why integrative and functional practitioners will often prioritize resolving constipation. Bulk laxatives, stool softeners, and stimulants can be useful as a short-term strategy but are only a band-aid. It’s important to understand the underlying causes and address it: slow motility and dysbiosis.
Restoring stool frequency and consistency to ideal can be a challenging and time-consuming process with involves multiple aspects of the 5R protocol. Useful tools include supplements like magnesium citrate, triphala, Swedish bitters, electrolyte replacement and hydration, increasing fiber (dietary and supplemental) as well as stimulating the migrating motor complex and balancing the HPA axis (stress response) through lifestyle modifications including exercise, massage, gargling and stress management.
Bottom Line
The second step in an individualized comprehensive gut restoration protocol involves promoting improved nutrient status and motility by exogenously replacing the necessary enzymes and nutrients needed. This is often done simultaneously to the Remove step, but in some cases, it’s addressed in sequence. It’s important to work with an integrative or functional medicine provider trained in the comprehensive gut restoration protocol to help you navigate this successfully.
Next, we will tackle the third “R” in the gut restoration protocol: Reinoculate.
CoQ10 and Kidney Health
Coenzyme Q10 (CoQ10), also known as ubiquinone, is an often forgotten about fat-soluble nutrient and key factor in optimizing kidney health. It can be made by our own cells when there are adequate resources, however it is also available through diet and as a supplement. In this blog we will discuss the association between CoQ10 and kidney health.
What is CoQ10 (what are the problems associated with its deficiency)
CoQ10 is naturally found in our cells with a structure similar to that of vitamin K. The main purpose of CoQ10 is in the mitochondria (energy factory of the cell) for production of Adenosine Triphosphate (ATP – the energy currency used to power all metabolic function), the supply of ATP is essential for every function of the body. Therefore, CoQ10 is extremely important for maintaining the health of every tissue and organ system of the body, including the kidney.
That said, CoQ10 is abundant in the most metabolically active tissue, which includes the heart, kidney, and liver. The concentration of CoQ10 in the kidneys are second only to the amount found in heart muscle. This makes perfect sense since these tissues would need the most energy. We would also expect that dysfunction in the production of CoQ10 would be linked with diseases of these organs.
The tubules of the kidneys are especially high energy consumers. They are involved in reclaiming many electrolytes and minerals lost during the filtration process by the kidneys. Therefore, they have a high concentration of mitochondria and high requirement for CoQ10.
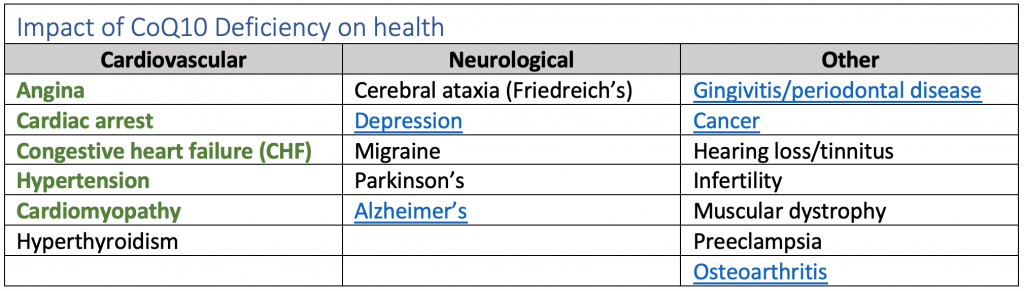
The table below outlines all the ways that CoQ10 deficiency impacts health (those linked with kidney disease complications are emphasized):
CoQ10 is a powerful antioxidant, which prevents the generation of free radicals responsible for damage to proteins, lipids, and DNA causing disease. In disease conditions connected with reactive oxygen species (ROS), like cardiovascular disease, diabetes, and kidney disease, the concentration of CoQ10 in the body is lower.
This is because the decline in production/circulation of CoQ10 leads to dysfunction of energetic production, which in turn decreases the efficiency of cells. To protect the cells against ROS, humans have evolved a highly complex antioxidant system, which includes CoQ10, glutathione, vitamin E, to name a few.
How is Coq10 related to kidney health?
Most of the body’s daily CoQ10 requirements come from endogenous synthesis, which is known to decline with age. Inflammatory mediators associated with kidney disease include C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) have also been shown to be reduced when deficient CoQ10 levels are corrected, indicating there’s a correlation between CoQ10 and inflammatory factors. The damage is thought to be mediated by the activity of nuclear transcription factor NF-kappa beta (NF-KB).
Decreased CoQ10 levels may be in particular an issue in Chronic Kidney Disease (CKD) patients prescribed statins. The kidney relies on a large amount of CoQ10 and ATP production in order to do its job of removing uremic toxins. Since CoQ10 depletion is a known side effect of statin therapy, it has been suggested that these patients have underlying mitochondrial disease leaving them more susceptible to the adverse effects of statin therapy.
Although hemodialysis is essential for removing uremic toxins in advanced stages of CKD, a consequence of the procedure is that patients are subject to additional oxidative stress (a result of neutrophil exposure to the synthetic material comprising the dialyzer membrane), in addition to the oxidative stress associated with CKD to begin with.
Furthermore, patients with CKD are at high risk of developing cardiovascular disease (CVD) compared to those without CKD. In fact, approximately 50% of CKD mortalities are a result of CVD and not a direct consequence of kidney failure. At the same time, CVD promotes CKD contributing to a vicious cycle.
Improving levels of CoQ10 resulted in improvements in blood cholesterol, markers of CV risk as a result of oxidative stress and therefore improves progression of CKD as a secondary end point. Furthermore, CoQ10 levels are associated with two other risk factors for CVD, higher epicardial fat thickness and low coronary flow reserve, a marker for atherosclerosis were found to be associated with reduced plasma CoQ10. Lastly, CoQ10 is significantly lower in hemodialysis patients, like many micronutrients.
Drug and Nutrient interactions Impacting CQ10 Deficiencies
CoQ10 deficiency is attributed to increased need outpacing production/supply due to mitochondrial dysfunction and/or increased oxidative stress exhausting antioxidants like CoQ10. As mentioned earlier, the increased demand in CKD due to elevations in uremic toxins and ROS is a drain on stores of CoQ10.
Coupled with dietary factors that affect consumption or absorption of foods rich in CoQ10 and cofactors needed to endogenously produce the nutrient, there are other influences to consider. For one, associations between drugs and nutrient deficiency are well documented. Drugs implicated with Drug-Induced Nutrient Depletion (DIND) of CoQ10 include beta-blockers, gemfibrozil, statins, warfarin, as well as certain chemotherapy and HIV medication.
Conversely, certain nutrients have been associated with improved efficiency. For example, carnitine, when co-administered, improves the efficacy of both CoQ10 and carnitine and improved circulation and protect the cardiac muscle. Another example is the synergistic antioxidant and antiinflammatory effect when CoQ10 is combined with vitamin E repletion. Furthermore, depletions in nutrients required for the production of endogenous CoQ10 will also affect the cellular production of CoQ10 via the Electron Transport Chain (ETC). These nutrients include iron, niacin (B3), vitamins B2, and B6.
Sources of CoQ10 (food and supplements, positive nutrient interactions)
Food sources of CoQ10 include:
· Meat and poultry, particularly organ meat and eggs.
· Fatty fish like Trout, herring, mackerel, and sardines.
· Legumes, especially soybeans, lentils and peanuts,
· Nuts and seeds, especially pistachios and sesame seeds
· A handful of fruits and vegetables that include strawberries and oranges, spinach, cauliflower, and broccoli)
Summary of Benefits of ensuring adequate CoQ10 on Kidney Disease
Adequate CoQ10 levels have been shown to be beneficial to slow progression of CKD by addressing direct and indirect factors:
· Improved Lipid profile (total cholesterol and LDL)
· Improved insulin sensitivity, glycemic control, and vascular dysfunction in type II diabetes
· Antioxidant protection from Inflammation
· Energy production in metabolically active tissue of the kidney
Vitamin K in Vascular, Kidney and bone Health
Vitamin D gets a lot of attention these days, not only for its roll in bone health, but also due to the multiple health benefits associated with robust levels of circulating vitamin D, specially in kidney disease. However, the role of vitamin K working in tandem with vitamin D often gets overlooked. This blog will focus on the importance of vitamin K in kidney health.
Vitamin K in kidney health
Nutrients don’t work solo; they function as part of a symphony with other nutrients to help our body perform at its best and prevent disease, including kidney damage and chronic kidney disease (CKD).
Vitamin D Background
Vitamin D3, also known as cholecalciferol, is a fat-soluble vitamin that plays a role as a pro-hormone. It Is naturally found in some foods like fish, egg yolks, and beef liver, but humans rely on making it through a process in the skin that requires sunlight exposure. For a full review of vitamin D, visit our previous blog.
Vitamin D plays a role in multiple aspects of health, including bone mineral balance, neuromuscular function, immunity and autoimmune disease, cognitive health, and insulin regulation. Maintaining adequate vitamin D levels can be beneficial when managing or treating many conditions, including:
- Back or musculoskeletal pain
- Osteoporosis
- Frequent colds or flu
- Cardiovascular disease
- Diabetes
- Fatigue
- Seasonal Affective Disorder (depression)
Vitamin D interaction with other nutrients
Calcium and Phosphorous
Vitamin D is necessary for the absorption of calcium and phosphorous from food sources in the intestines into the blood as well as regulating the amount of those minerals in circulation. This is a delicate balance. These two minerals are essential for bone remineralization and remodeling (bone building), skeletal muscle contractility, as well as kidney and cardiovascular health.
Vitamin K: K1 vs. K2
Vitamin K is a general term for a group of compounds that function as part of the coagulation (blood clotting) pathways that help us regulate blood thinning and prevent the risk of bleeding from cuts or injuries. Vitamin K is typically thought of for bleeding disorders, but the proper balance may also be useful in the management and treatment of the following conditions:
- Atherosclerosis and ischemic heart disease
- Cirrhosis, hepatitis, and liver disease
- Osteoporosis ad bone loss
- Cancer
Vitamin K naming and structure is one of the most misunderstood topics in nutritional medicine, so let’s spend a few minutes understanding these foundations. Vitamin K is the general term used to describe a group of compounds that share a common ring structure. There are 2 subcategories to keep in mind, K1 and K2.
K1, also known as phylloquinone or phytonadione, is the primary form found in plants. Leafy vegetables are the main source of vitamin K1. This is the form that is utilized in the liver and involved in clotting. This is also the form that interacts with blood thinners like warfarin and why patients on blood thinners are told to carefully monitor their intake of leafy greens.
K2, referred to menaquinones, describes a group of varying chemical structures that are used outside the liver (in the bone and vascular wall). The number of isoprenyl units determines the name of the structure. So, a K2 with 4-isoprenyl units is MK4, and MK7 means there are 7 isoprenyl units. This is the form that is involved in bone health and the focus of kidney-relevant associations of this vitamin.
Vitamin K2 can be found in the diet in multiple forms (for example, MK4, MK7, MK8, and MK9) in egg yolks, meats, cheese, and other dairy products, natto (fermented soybeans), and seems to be much more readily bioavailable than K1 sources. In addition, menaquinones are synthesized by bacteria in the colon.
Considering what we already know about the impact of the microbiome on kidney health and risk factors, menaquinone might play a larger role in the gut-kidney connection than we’ve been giving it credit for. Though there are multiple factors that contribute to the circulating level of vitamin K in the body, there is speculation that dysbiosis (imbalance of healthy gut bacteria in the gut) may be a significant contributing factor to inadequate circulating levels of K2 contributing to cardiovascular, kidney, and bone disease.
Supplementing Vitamin K2
Supplements containing vitamin K are available commercially as K1, MK4, and MK7. However, it’s important to note that the safety and efficacy of each form are not equal and have contributed to significant confusion and inaccurate recommendations.
There’s little evidence supporting the supplementation of phylloquinone or K1. This may be due to poor bioavailability and a low rate of conversion to active forms. K2, on the other hand, seems to be the superior form, with more recent data supporting the use of MK7.
In older studies, the MK4 form seemed to be established as the most efficacious form when it comes to certain conditions like osteoporosis and hepatocellular carcinoma. However, MK7 seems to be more bioavailable and has a steadier blood concentration when compared to MK4 (and K1), meaning more stable blood concentrations and requiring lower doses. Additionally, the added benefit of MK7, particularly in osteoporosis, may partly be related to MK7 inhibition of NF-kB, an inflammatory mediator known to increase disease progression.
Like Vitamin D3, K2 helps to manage calcium deposition. Adequate levels of Vitamin K2 are required to deposit calcium into the bone and to prevent calcium deposition into soft tissue like cardiovascular arteries and the kidneys. So, you see, depletion of K2 along with vitamin D3 can be a risk factor for bone loss, cardiovascular and kidney disease and supplementation of the two combined might yield the best health outcomes.
Manufacturers and companies selling vitamin K supplements often do not reveal whether contains MK-4 or MK-7. Therefore, it’s important to use formulations with full transparency and quality control. This is important for ensuring the highest efficacy but also for safety concerns surrounding vitamin K supplements and that they may interfere with the stability of anticoagulant medications such as warfarin. This is also why it’s very important to work with your nutritionist or doctor to choose the correct product and dose. If you’re on a blood thinner, never make changes to your diet or supplements without consulting with the healthcare provider monitoring your medication and INR (test for bleeding tendencies).
Studies that examine the benefit of MK-7 supplementation showed a positive effect on cardiovascular calcification. Although MK-7 did not prevent CKD-associated hypertension and hypertrophy, it did demonstrate the prevention of calcification of blood vessels. The recommended dose of MK-7 is typically 45μg/d, though doses of 100μg/d have been suggested, likely based on studies of the less potent form of MK-4 which, as explained above, is not equivalent.
Although further studies are still underway, it’s safe to say that considering the low risk and cost, it’s worth optimizing vitamin D3 and K2 (MK7) levels in patients with associated risk factors for or diagnosed with CKD. Work with your nutritionist and nephrologist to personalize your intake and optimize your nutrient levels safely and effectively.
Pharmacogenomics: Advances in Individualized Treatment in Kidney Disease
According to a report by the US Food and Drug Administration (FDA), 2.2 million adverse drug reactions (ADRs) occur in the US annually. On the other hand, the healthcare cost and morbidity associated with ineffective medication cause unnecessary burdens as well as patient confidence in treatment. Clinicians continuously strive to strike a balance between medication choices and doses that are too high and cause toxic side effects and those that are dosed too low and become ineffective. Here, we will discuss the role of pharmacogenomics in prescribing for kidney patients.
By Lara Zakaria, RPh MS CNS CDN IFMCP
Variability in response to pharmaceutical therapy is due to multiple, complex individual variations in basic physiological differences like age, sex, and weight, as well as metabolism, absorption, organ function, and disease state. These variables mean patients require monitoring, dose adjustments, and sometimes alternative therapy (drug switch).
This is why medications come in various doses, formulations, and options exist within a drug class. Prescribers follow “best practice guidelines” that allow them to choose an alternative when one drug therapy fails or is not tolerated due to side effects or ADR. But even with these well-researched guidelines, the choice and dosage of a medication can still be a guess.
Wouldn’t it be a relief to be able to predict in advance how someone might respond to a medication, avoiding guessing, saving time and improving patient outcome?
Thanks to advances in a field of genetics called pharmacogenomics (PGx), clinicians are starting to use genetic information to personalize drug therapy. Though this has a broad range of benefits, our focus here will be how this impacts those who have kidney disease (KD) or kidney transplant.
Defining Pharmacogenomics
DNA is made up a sequence of four nucleotides, (adenine, cytosine, guanine, thymine). The genetic sequence serves as a template which our cells use it to make thousands of proteins which are essential for carrying out all biological functions that maintain life. This includes the enzymes, receptors, and channels that are involved in the absorption, transport, and metabolism of drugs.
Alterations in the sequence, like single nucleotide polymorphisms (SNPs), or errors in the original code, may affect the efficiency of protein production. In turn, this may alter the efficacy or toxicity of a medication for an individual with that SNP.
When scientists began exploring pharmacogenetics in the 1950’s, clinical research focused on connecting single genetic variations with a predictable outcome in drug therapy. In the last decade and a half, it has lead to an evolution of pharmacogenomics, exploring the complex interplay of genetic and environmental influences on individual genetic expression.
Aside from being a relatively new area of focus, this nuanced difference is what makes “omics”research more challenging to translate into clinical application. Even though we’re only at the tip of the proverbial iceberg of this revolutionary approach to individualized medicine, useful clinical implications are already emerging, in particular when it comes to medications often used in kidney patients.
Pharmacogenomics of kidney disease management
To date, there are approximately 300+ medications with FDA-approved PGx labeling recommendations. Of these, a handful are common in kidney disease patients. These categories include*:
- Cardiovascular Disease (CVD) – including clopidogrel (CYP2C19), simvastatin (SLOCO1B1) among other HMG-COA inhibitors, also known as “statins,” and warfarin (multiple, including CYP2C9, CYP4F2, VOKORC1, and potentially APOE, ABCB1, and UGT1A1)
- Transplant medications – including azathioprine (TPMT), tacrolimus (CYP3A4/3A5), and the antifungal agent voriconazole (CYP2C19)
- Hyperuricemia (elevated circulating uric acid) – allopurinol (HLA-B)
- Diabetes, with metformin being the most well-known (SNP rs11212617).
| Therapeutic Category | Drug | Genetic SNP |
| Cardiovascular Disease (CVD) | Clopidogrel | CYP2C19 |
| HMG-CoA inhibitors (statins) | SLOCO1B1 | |
| Warfarin | CYP2C9, CYP4F2, VOKORC1, and potentially APOE, ABCB1, and UGT1A1 | |
| Transplant medications | Azathioprine | TPMT |
| Tacrolimus | CYP3A4/3A5 | |
| voriconazole (antifungal agent) | CYP2C19 | |
| Hyperuricemia (elevated circulating urea) | Allopurinol | HLA-B*58:01 |
| Diabetes | Metformin | SNP rs11212617 |
* This is a list narrowed down limited to strength of available evidence and relevance to KD by Adams et al.
The need for individualized therapy in kidney patients through pharmacogenomics
Even when dosing is in line with best practice guidelines, they may still be sub-optimal or nephrotoxic (toxic to the kidney). Either situation for a transplant or KD patient may be life-threatening. Knowing in advance how a patient will respond to a therapy means avoiding the cycle of “guess, assess and adjust,” saving valuable time.
Let’s use the medication tacrolimus as an example. Tacrolimus is a very widely used and preferred immunosuppressant medication used in patients after a kidney transplant. Monitoring is very important because there’s a small margin of error for dosing. Due to this narrow therapeutic window, a little too much of the drug can quickly result in toxicity, while slightly under-dosing will be ineffective and potentially cause transplant rejection. Either situation can be life-threatening for a transplant patient.
Tacrolimus is metabolized by enzymes (part of the cytochrome P450 class) referred to as CYP3A4 and CYP3A5. It’s understood that individuals considered “hyper-metabolizers” – enzymes work faster than average to metabolize (break down) the drug - require higher dosing to achieve therapeutic efficacy. Meanwhile, slow metabolizers require smaller doses to maintain safe and effective therapeutic levels. Because of the narrow therapeutic window, there is a small margin for accuracy, making these variations in metabolic functions particularly relevant to safety and outcome.
It’s interesting to note that there are various factors that influence CYP3A4/3A5 rate of metabolism. Use of other common medications like acetaminophen, clarithromycin, SSRI antidepressants (like fluoxetine and sertraline), and even caffeine, cannabis, and phytonutrient compounds naturally found in foods and herbs can also serve to up or downregulate the function of CYP3A4/3A5– further confounding or contributing to successfully achieving the narrow therapeutic window.
Another interesting example if the case of a transporter gene SLCO1B1 and its effect on statin therapy. This anti-cholesterol medication class (which includes simvastatin, rosuvastatin, pravastatin, etc) is well known for an ADR which causes muscle breakdown, rhabdomyolysis. Risk increases with increased blood concentration or dosage. Furthermore, muscle breakdown can add stress on the kidney.
SLOCO1B1 is responsible for transporting the drug out of the blood and into the liver for detoxification. In individuals with genetic variations that reduce the function of the transporter, accumulations are more likely to occur, and rhabdomyolysis is more likely even at lower drug dosage than typically expected. Combine this with environmental factors or combination drug therapy that may further slow detoxification, liver function, or drug clearance, and you can start to see how multiple factors may contribute to presentations of this ADR.
Should you get genetic testing?
There are a multitude of consumer tests offering direct to consumer genomic and health data like 23andMe, among others. The health reports are limited, and may omit some crucial lifestyle and epigenetic factors, but can be a good starting point. Raw SNP data can be obtained, but interpretation requires a strong understanding of clinical significance so working directly with a healthcare professional with appropriate training is strongly recommended.
There are also professional tests and reports available through your health care provider like IntellexxDNA and MyDNA. Your PGx-literate provider can help you choose the appropriate test, and most importantly guide you in recommendations once you have your results in hand.
It’s essential to remember that the field of PGx is relatively young and complex. When we talk about genetics, we cannot ignore the significance of epigenetics. In a previous blog, we discussed how dietary and lifestyle modifications can affect expressions of these genetic traits.
Another related emerging field is nutrigenomics– a cousin to PGx - focusing on the effect of nutrients on genetic expression. When interpreting emerging findings in omics, clinicians must take into consideration the effect of factors like nutrition, herbal use, as well as environmental toxins, and medication on epigenetics expression. The individualized and integrative approach to managing kidney disease will rely on the nephrologists, pharmacists, and clinical nutritionists of the not-so-distant future working together to layer those factors to maximize therapeutic benefit and reduce harm to patients.
Epigenetics: Understanding the new research in genetic risk of kidney disease
This is a beginning of a series of blogs that explores the emerging view regarding the genetic risk of kidney disease. Consider this series your crash course in epigenetics! We will also discuss epigenetics in kidney disease. But before we dive in, let’s start with a refresher on genetics.
By Lara Zakaria, PharmD CNS CDN IFMCP
You may remember from your high school science class that DNA is a long and windy spiral made up of millions of nucleotides. There are 4 nucleotide bases (adenine, cytosine, guanine, thymine), the sequence of which determines how genetic traits are expressed.
The nucleotide sequence is the foundation of about 20,000 genes. Our cells read the genetic sequence and use it to make thousands of proteins that are essential for carrying out biological functions that maintain life.
Alterations in the sequence, like single nucleotide polymorphisms (SNPs), or errors in the code, affect the efficiency of the protein production process. These may sometimes lead to disease or increased risk for disease. Interestingly, on occasion, they may have no effect on risk and may even be protective against disease.
Genetics and disease risk, the old view
For decades, scientists assumed that if we were to interpret the DNA sequence, we’d be able to “crack the code” and unlock the potential to identify and repair “broken code” and prevent or reverse disease. This was an exciting prospect; however, as it turns out, there’s actually another way.
Since the early 2000’s researchers began to notice that people with so-called “bad genes” weren’t expressing disease the way we would have predicted, and a new theory began to shape the paradigm of genetics. What if lifestyle, environmental influences, and emotional factors played a role in genetic expression? This was the beginning of the emergence of a new field of research called epigenetics.
What is Epigenetics?: The “protein factory”
If epigenetics had a tagline, it would be: Your genes are not your destiny.
Recall that the nucleotide sequence of DNA codes for the production of proteins that are used for thousands of biological processes in the body. So, we’ll call this the “protein factory.” Now, imagine that your DNA acts like a conveyer belt and that there’s a scanner that reads the DNA sequence, interpreting the instructions for protein production.
Now, let’s imagine there’s a factory worker that sits at a switchboard. He’s in charge of a lever that controls the speed of the “conveyer belt” and a button that can turn the scanner on or off. Our factory worker can speed up the belt to produce proteins faster or turn it down to make less. He can also decide to turn off the “scanner,” so it skips certain genes and doesn’t make that particular protein (maybe there’s already or much of it, and we don’t need more).
Though it’s a rough example, this is essentially how epigenetics influence genetic expression, protein production, and in turn, whether a genetic risk is expressed (leading to disease) or suppressed (not getting the disease). In other words, in our example, factors that influence disease risk are “the boss,” who instructs our “protein factory” worker on how to operate the lever and scanner.
That’s why we summarize this evolved understanding of genetics to say, “Our genes are not our destiny.” We now understand that they are not set in stone at birth, determining our fate. Our lifestyle and environment (the boss) help to shape how your epigenetics (the factory worker at the switchboard) expresses your health outcomes.
Here’s a key takeaway: Just as much as good lifestyle habits can reduce disease risk by turning off problematic genes, our poor decisions can increase our disease risk.
How epigenetics changes the conversation about disease risk?
You can probably start to see how these revelations about the role of lifestyle and environment have completely changed the conversation about the genetic risk of disease. Scientists who once thought that we have to literally change our genes to modify disease risk are now exploring how to use epigenetic influence to improve health outcomes.
Lifestyle factors like diet, sleep, meditation, and exercise are things we have control over and have a tremendous influence on genetic expression. Limiting exposure to environmental toxins (like food additives, pesticides, heavy metals, and plastics) and supporting the body’s natural detoxification pathways is another essential aspect to keep in mind.
Ever wonder how relatives that haven’t met or spent little time together may express similar personality traits or likes and dislikes?
Well, epigenetics may even play a role in the expression of traits like personality, stress resilience, and even likes and dislikes (like food!). As it turns out, the experience of past trauma may also be passed down as a result of epigenetics.
So, think of ancestors who survived famine, war, or torture – we now realize their experiences can influence genetic expression. There’s even evidence that experience during pregnancy can influence a baby’s risk later in life epigenetically.
Could this be part of the reason why certain populations are more prone to certain chronic diseases? Future research may tell us more.
The role of epigenetics in kidney disease
The field of epigenetics is still in its early phases, and the publications exploring the relationship to kidney disease specifically are even younger. However, there’s an exciting and growing body of research exploring these ties.
Furthermore, the breakthroughs in the fields of cancer and aging are very encouraging and give us insight into what might be successful for kidney disease. As we learn more, this emerging research may help us to slow disease progression and potentially even reverse kidney disease in the near future.
References
- Weinhold, B. Epigenetics: the science of change. Environ Health Perspect. 2006;114(3):A160-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1392256/
- Mazzio EA, Soliman KF. Basic concepts of epigenetics: impact of environmental signals on gene expression. Epigenetics. 2012;7(2):119-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335905/
- Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med. 2012;34(4):753-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3515707/
- Dwivedi, Rama S. et al. Beyond genetics: epigenetic code in chronic kidney disease. Kidney International, 2011; 79(1): 23 – 32 https://www.kidney-international.org/article/S0085-2538(15)54674-7/fulltext
- Wanner N, Bechtel-Walz W. Epigenetics of kidney disease. Cell Tissue Res. 2017 Jul;369(1):75-92. Epub 2017 Mar 13. https://www.ncbi.nlm.nih.gov/pubmed/28286899
Inflammation, Leaky Gut and Kidney Disease
Researchers have found exciting links between diet, lifestyle and the risk for kidney disease (KD). The most significant relationship is between the health of the gastrointestinal (GI) tract and the risk of KD. This relationship is referred to as the gut-kidney axis. Various factors, including diet and exposure to toxins, can compromise the integrity of the lining of the intestines. This can lead to intestinal permeability, more commonly called leaky gut.
What is Leaky Gut
A normal and healthy GI tract has a natural barrier. This barrier serves to protect the intestines and ensure proper absorption of nutrients and elimination of toxins. This barrier also serves to protect the integrity of the microbiome – the “good” bacteria that lives in our GI tract and works with our body to maintain good health.
As the phrase implies, leaky gut describes when the cells that make up the lining of the GI tract separate enough to allow the contents of the gut to leak out. This is a problem because it reduces absorption of nutrients, causes toxins to build up, and results in systemic inflammation.
Inflammation is a response of the immune system that treats the leakiness as a threat. This increased inflammatory response has been linked to increased risk of many chronic diseases, including diabetes and kidney disease, among others.
Diet and Leaky Gut
Now that we understand that factors that compromise the intestinal barrier can lead to systemic inflammation and kidney disease, let’s outline the factors that lead to leaky gut.
Processed foods and high-sugar foods
Think of processed foods as most foods that are altered from their natural state and packaged. Processed foods include bread, cereals, lunch meats, and even protein bars. The processing of these foods usually strips the nutrient content of the ingredients in their whole, “live” form.
Along with depleting nutrients, this also often adds to the glycemic load, spiking blood sugar and insulin levels contributing to diabetes and metabolic disease. Along with high sugar foods, like soda, fruit juice, and sweets this significantly contributes to inflammation and leaky gut.
In fact, kidney disease patients who consumed a “pro-inflammatory” diet had 1.55 times increase in the risk of going into kidney failure requiring dialysis than those who did not. This diet included tomatoes, carbonated beverages, processed meat, red meat, organ meat and fish other than dark-meat fish.
Food sensitivities
Food allergies can be very serious and life-threatening. They can start off as a rash or hives, and progress to shortness of breath and difficulty breathing. These are not to be confused with food sensitivities, which are less severe are usually not life-threatening. However, they do increase inflammation and contribute to leaky gut.
Common food sensitivities include gluten, dairy, soy, peanuts, corn, and eggs. For many people who have sensitivities to these foods, even a short-term elimination can help reduce inflammation and heal leaky gut.
Caffeine and alcohol
Regular consumption of caffeinated beverages and alcohol is irritating to the GI tract. Over time, this compromises the lining of the intestines. Tolerance varies for each individual based on genetics and other factors, so universally safe guidelines are difficult to establish. In general, it’s best to reduce consumption or remove these triggers at least for short elimination period.
Eating the wrong fats
Just like processed carbohydrates can be damaging to the gut, processed fats are also inflammatory. The good news is that consumption of anti-inflammatory fats, can help to reverse this process This includes sources of polyunsaturated fats like olive oil, omega 3 fats found in fish, nuts, and avocado.
Certain medications
Certain medications can be very irritating to the intestinal barrier. Common examples include:
• NSAIDs – non-steroidal anti-inflammatory like ibuprofen, aspirin, and naproxen usually used to treat pain and fever. Regular use of these medications (both prescription and over-the-counter) is highly associated with increased incidence of leaky gut.
• Steroids like prednisone or methylprednisone, often prescribed for inflammation or asthma, like NSAIDs, are damaging to the lining of the gut.
• Antibiotics disturb the natural balance of good bacteria in the gut. Courses of antibiotics kill off good bacteria along with the bad bacteria causing the problematic infection. This leads to dysbiosis, increased inflammation, and leaky gut.
If you are taking these medications, it’s important not to discontinue or alter your medications without speaking to your physician first. For more information, please see our blog on medication and kidney health. If you are concerned about leaky gut, you can work with an Integrative or Functional Medicine practitioner to help you manage your medications and heel your intestinal permeability.
Leaky Gut and Kidney Disease: 6 Classes of medication that might be contributing
Prescriptions and over-the-counter medications can be used safely and effectively to help treat a long list of problems. However, they may also have side effects that contribute to a “leaky gut” which may increase the risk of kidney disease, as well as other chronic diseases. In this blog, we will discuss leaky gut and medications.
Antibiotics
It is well known that antibiotic use leads to significant disruption in microbial balance. This leads to dysbiosis and subsequent disruption of the protective layer in the gut wall – commonly called “leaky gut” or intestinal permeability.
The “leaks” in the gut may bacteria DNA and undigested food to leak into the bloodstream contributing to inflammation and disease progression. You can learn more about that in our blog on inflammation.
NSAIDS
Nonsteroidal anti-inflammatory drugs or NSAIDs include common over-the-counter medications like ibuprofen and naproxen as well as prescription-strength diclofenac, meloxicam, and celecoxib. These are usually used to help reduce pain and swelling associated with inflammation.
Aspirin
An NSAID used by millions for the prevention of heart attack and stroke, often in low doses, is Aspirin. Kidney patients are at higher risk for heart disease and many take low-dose aspirin. Aspirin works to reduce heart disease risk by reducing inflammation and blood-thinning; however, it’s generally considered safe and beneficial in low doses.
At a very low dose, the side effects may be less pronounced than the effects of regularly using higher dose NSAIDs for pain relief. However, studies show that aspirin increases intestinal permeability when taken at higher doses.
Acetaminophen
Acetaminophen is one of the most commonly used medications for pain and fever. It’s technically not an NSAID, but instead, works by a slightly different mechanism that’s considered safer regarding the risk of causing ulcers.
However, it has also been found to also contribute to a leaky gut. Furthermore, researchers found that it may also help promote the passage of unfavorable bacterial toxins into the bloodstream, promoting inflammation.
But that’s not the only concern with the chronic use of acetaminophen. The drug is well known to negatively affect liver function. This is because acetaminophen depletes glutathione (the body’s most important antioxidant) and significantly slows the liver’s ability to remove toxins.
Even though acetaminophen use has not been directly linked to kidney damage, the accumulation of toxins, depletion of glutathione, and increased intestinal inflammation can worsen or promote kidney disease in susceptible individuals
Steroids
Steroids, like dexamethasone, prednisone, or methylprednisolone, are also used as anti-inflammatory agents sometimes for more extreme cases of rheumatic pain. But they are also used in treating asthma, allergic reactions, or reducing inflammation in the lungs to improve breathing.
One would think that medications have anti-inflammatory properties and they would decrease inflammation in the gut. However, the opposite is true.
A well-known and common side effect of these medications are stomach ulcers and increased intestinal permeability. Studies have demonstrated that they cause disruption to the gut barrier in healthy individuals. In other words, the side effects of NSAIDs have been shown to contribute to disease progression in kidney patients by alterations in the integrity of the GI.
Proton Pump Inhibitors (PPI)
Proton pump inhibitors (like omeprazole, pantoprazole, esomeprazole, etc) are usually used for the treatment of stomach ulcers, reflux, and occasional heartburn. They work by reducing the stomach acid being made.
However, recently they’ve been found to cause kidney damage. Because these medications lower the pH (the acidity) of the stomach and small intestine, they cause food to not be digested properly, which leads to nutrient malabsorption. Also the change in the acidity of the stomach cause dysbiosis especially in the small intestines (known as small intestinal bacterial overgrowth or SIBO)
Phosphate Binders
Medications like calcium acetate, lanthanum, and sevelamer are in a class of medication we call phosphate binders. These are commonly prescribed by kidney doctors to prevent a common complication of kidney disease, elevated phosphorus. Phosphate binders work by blocking the absorption of phosphorus in the gut and preventing it from entering circulation. This protects the kidneys since elevations of phosphorous are connected with increased kidney damage.
Another interesting benefit of these medications is that they bind some bacterial toxins and prevent their absorption into circulation. Therefore, there are two mechanisms that protect the kidney from further damage.
However, recent research shows that these medications may change the intestinal balance of good bacteria which may contribute to further damage to the intestinal wall. More research needs to be done to determine the risk vers benefit of using phosphate binders in some cases.
Bottom Line
It’s a good idea for patients at risk of or managing kidney disease to understand the risks and benefits associated with your medications. If you are working with a Functional Medicine practitioner that can help you learn how to repair your gut wall and balance the gut bacteria, gut wall, reducing inflammation and helping you better manage your kidney disease.
Talk to your doctor or pharmacist if you have questions about your medication; never discontinue medication without speaking to your doctor.
References
J Clin Pharm Ther. 2003 Aug;28(4):289-94
Basic Clin Pharmacol Toxicol. 2015 Sep;117(3):195-203
Curr Pharm Des. 2015;21(35):5089-93
Cell Physiol Biochem. 2013;32(2):431-47
Kidney Int Suppl. 2009 Dec;(114):S12-9
The Gut-Kidney Axis: Nutrition Can Slow the Progress of Kidney Disease
The human gut is home to a significant number of bacteria that are essential for good health. In fact, in recent years, scientists have found a link between these bacteria and the risk of diabetes, heart disease, dementia, depression and chronic kidney disease. Here we will discuss what is described as the gut-kidney axis.
The ecosystem of bacteria lining the gastrointestinal (GI) tract is referred to as the microbiome. For the most part, these bacteria are beneficial, and contribute to our health and wellbeing. However, sometimes the wrong bacteria (or other organisms like yeast or parasites) can outgrow the “good” bacteria. When that happens, it’s called dysbiosis.
Dysbiosis and Kidney Disease: The Gut-Kidney Axis
Recent studies have demonstrated a close relationship between the quality of the microbiome and the health of the kidney. This relationship has been called the gut-kidney axis.
According to this research, those with a healthy microbiome, abundance of good bacteria and no overgrowth of bad bacteria, are less likely to develop chronic kidney disease. In fact, the presence of certain strains of bacteria in the gut can actually slow the progression of chronic kidney disease and even reduce the need for dialysis.
This is extremely significant because it means we can use dietary interventions to reduce the risk of a life-threatening disease that affects 20 million Americans.
Fiber and a Healthy Microbiome
For a healthy microbiome to flourish, the environment of the GI must be conducive to grow normal strains of gut bacteria. The composition and function of these bacteria depend on the available nutrients. Normal gut bacteria thrive when supplied with fiber. This “bacteria food” is called prebiotics. depend on fibers in the diet. For example, fruits and vegetables are great prebiotic sources.
But that’s not the only benefit of eating a high fiber diet. Fiber acts as a “net,” catching and removing toxins in the gut to make sure they don’t cause damage. This is especially significant in kidney disease where increased of toxins such as uric acid, oxalate and urea leads to worsening of the disease. This creates an environment where “bad” bacteria that feed on these toxins flourish in the colon (lower intestines) instead of the “good” ones that consume fiber.
Therefore, increased consumption of fiber-rich foods helps in two ways: First, it creates a net that helps to remove the excess toxins in the feces. Second, it helps to feed the beneficial bacteria and create an environment unfavorable for the growth of the “bad” bacteria.
Recommendations for a Healthy Gut
1. Focus on fiber and nutrient-dense foods. Reduce your intake of starchy veggies and eat more dark leafy greens (like spinach and romaine), cruciferous veggies (like broccoli, cauliflower, and kale) and fresh whole fruit (but limit to two servings per day).
2. Add food sources of probiotics to your diet, including fermented vegetables and drinks like raw sauerkraut, kimchi, pickles, apple cider vinegar, and kombucha. It’s recommended these are eaten raw because pasteurization process will destroy the bacterial content of these foods.
3. In general, reduce processed carbs, like bread, cereals, and high sugar foods. These types of foods are usually low in fiber and help to contribute to starving out the beneficial bacteria.
4. Drink plenty of water and ensure daily regular bowel movements.
Diet and lifestyle changes can be difficult to do and maintain on your own. We encourage you to seek out the support of a functional medicine-trained nutritionist that specializes in kidney disease and familiar with using food interventions to address the gut-kidney axis.
References
Cani PD, Osto M, Geurts L, Everard A. “Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity.” Gut Microbes, 2012: 3:4, 279-288, DOI: 10.4161/gmic.19625.
Kieffer, DA., et al. “Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats.” Am J Physiol Renal Physiol, 2016: 1;310(9):F857-71. doi: 10.1152/ajprenal.00513.2015.
Moraes, C., et al. “Trimethylamine N-Oxide From Gut Microbiota in Chronic Kidney Disease Patients: Focus on Diet.” Journal of Renal Nutrition, 2015: Vol 25, No 6: pp 459-465 http://dx.doi.org/10.1053/j.jrn.2015.06.004.
Ramezani, A., Raj, D. “The Gut Microbiome, Kidney Disease, and Targeted Interventions.” J Am Soc Nephrol, 2014: 25: 657–670.
Rossi M, et al. “The Kidney–Gut Axis: Implications for Nutrition Care.” Journal of Renal Nutrition, 2015: Vol 25, No 5; pp 399-403.
Rossi, M., et al. “Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial.” Clin J Am Soc Nephrol, 2016: 11: 223–231, doi: 10.2215/CJN.05240515.
Ulluwishewa, D. “Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components.” Journal of Nutrition, 2011: 269-776.