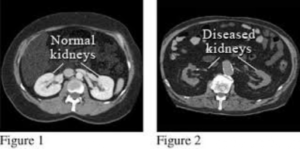
ما هو مرض الكلى المزمن؟
لم يتفق أطباء الكلى لفترة طويلة على تعريف مرض الكلى المزمن. وفي العام ألفين واثنين، نشرت المؤسسة الوطنية للكلى الامريكية أول إرشاداتها (مبادرة جودة نتائج مرض الكلى) التي حددت تعريف مرض الكلى المزمن. وقد وصف ذلك بأنه أي أذية كلوية تستمر لمدة ثلاثة أشهر أو أكثر. و تم تعريف الأذية الكلوية بأنها واحد من الاحتمالات التالية
(١. هبوط وظيفة الكلى لما يقل عن ستين ملليتر/دقيقة (أو ستين بالمائة كما أفضل أن أطلق عليها، حيث يتجاوز الأداء الطبيعي للكلى تسعين ملليتر/دقيقة
٢. اختبارات البول غير الطبيعية التي لا تتحسن خلال ثلاثة أشهر
(٣. أن تكون بنية الكى غير طبيعية عند التصوير الشعاعي (مثل الإيكو، التصوير الطبقي المحوري، إلخ
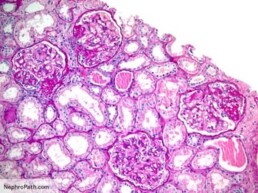
مرض الكلى المزمن
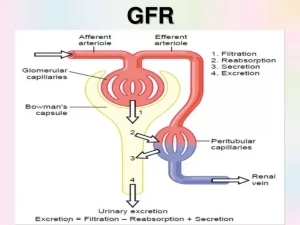
تطرح أجسامنا فضلات في مجرى الدم نتيجة لتحلل الغذاء و الاستقلاب و عمل الأنسجة. يتم إزالة العديد من هذه الفضلات من مجرى الدم بواسطة الكلى. يطلق اسم معدل الرشح الكبيبي على الرقم الذي يقيس مدى قدرة الكلى على القيام بوظيفتها، و هو مقدار الفضلات التي تتم إزالتها من قبل وحدات الترشيح الكلوية (النفرونات) كل دقيقة
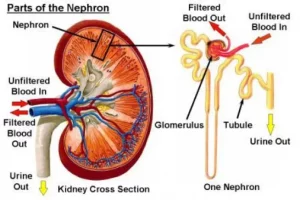
معدل الرشح الكبيبي
الكرياتينين هو أحد هذه الفضلات و هو يأتي من العضلات. يساعد النظر على مستوى الكرياتينين في الدم على تحديد معدل الرشح الكبيبي. يتم طرح بعض الكرياتينين في الأنابيب الكلوية، مما يجعل الكرياتينين مشعر غير مثالي لتحديد معدل الرشح الكبيبي
تقدير معدل الرشح الكبيبي
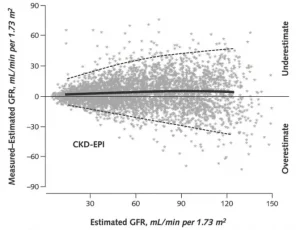
تم تطوير العديد من المعادلات التي تستخدم مستوى الكرياتينين في الدم، والجنس لتقدير معدل الرشح الكبيبي. يتم استخدام الجنس بسبب الاختلافات الطبيعية في كتلة العضلات التي تنتج الكرياتينين. المعادلة التي تستخدم أكثر من غيرها تسمى معادلة التعاون الوبائي لأمراض الكلى المزمنة. بامكانك مراجعة هذه المعادلة من خلال هذا الرابط

و لكن هذه المعادلات تكون أكثر دقة عندما تكون وظائف الكلى منخفضة. في حين كلما كانت وظائف الكلى أعلى كلما انخفض أداء المعادلات و ازدادت أخطاؤها كما يبدو في الرسم البياني أدناه. بالإضافة إلى ذلك، تزداد أخطاء هذه المعادلات إذا كان الشخص في سن متقدمة، أو زائد الوزن، أو لديه حجم كبير من العضلات كرافعي الأثقال مثلاً
كلما ارتفعت وظائف الكلى كلما انخفض أداء المعادلات و ازدادت أخطاؤها
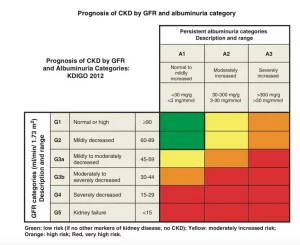
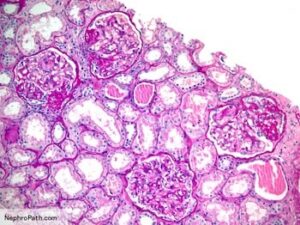
تصنيف القصور الكلوي
وقد قسمت الإرشادات أيضا مرض الكلى إلى خمس مراحل حسب مستوى وظيفة الكلى. المرحلة الأولى هي عندما يكون معدل الرشح الكبيبي يساوي أو أكثر من تسعين. في حين أن المرحلة الخامسة هي عندما يصبح معدل الرشح الكبيبي أقل من خمسة عشر. و في العام ألفين وإثني عشر، تم تقسيم المرحلة الثالثة من معدل الرشح الكبيبي إلى المرحلتين; المرحلة الثالثة ألف و المرحلة الثالثة باء. وقد أضيفت أيضًا كمية البروتين في البول إلى التصنيف لأن مستوى البروتين في البول يساعد على تقدير احتمال تدهور وظيفة الكلى على مر الزمن
(المرحلة الأولى: أذية كلوية مع معدل رشح كبيبي طبيعي (تسعين أو أكثر مليليتر/دقيقة/١.٧٣ متر مربع
(المرحلة الثانية: أذية كلوية مع انخفاض طفيف في معدل الرشح الكبيبي (من ستين إلى تسعة وثمانين مليليتر/دقيقة/١.٧٣ متر مربع
(المرحلة الثالثة: أذية كلوية مع انخفاض متوسط في معدل الرشح الكبيبي (من ثلاثين إلى تسعة وخمسين مليليتر/دقيقة/١.٧٣ متر مربع
(المرحلة الرابعة: أذية كلوية مع انخفاض شديد في معدل الرشح الكبيبي (من خمسة عشر إلى تسعة وعشرين مليليتر/دقيقة/١.٧٣ متر مربع
(المرحلة الخامسة: فشل كلوي (أقل من خمسة عشر مليليتر/دقيقة/١.٧٣ متر مربع أو على التحال الكلوي
تصنيف القصور الكلوي حسب معدل الرشح الكبيبي و مستوى البروتين في البول
في المدونات القادمة، سنتحدث عن وباء مرض الكلى المزمن ومنهجنا المتكامل والفردي لتقديم الرعاية الطبية المتكاملة لمرضى الكلى
Pesticides and Kidney Problems: An Unseen Link?
Kidney disease is a serious health problem that affects 1 in 7 people in the USA. Because of the elaborate way the kidneys filter the blood of waste products, they end up getting exposed to higher concentrations of environmental toxins. Pesticides are chemicals that farmers use to protect crops from pests. They, too, can affect the kidneys. This blog will discuss the connection between pesticides and kidney problems.
By Majd Isreb, MD, FACP, FASN, IFMCP
Pesticides: What Are They?
Pesticides are chemicals that farmers use to protect crops from pests like insects, rodents, and weeds. These pests can eat or damage the crops, leading to less food production. To ensure they have a successful harvest, farmers often use pesticides.
Pesticides are not just one type of chemical. They come in many forms and can be used to control a wide variety of pests. For example, insecticides kill insects, herbicides kill weeds, and rodenticides kill rodents. They can be sprayed on crops, added to soil, or even applied to seeds before they're planted.
Pesticides and Health
While pesticides play a big role in helping produce enough food, these chemicals can also be harmful. Pesticides are designed to kill or repel living organisms, and unfortunately, they can affect other organisms, too, including humans.
When humans are exposed to pesticides, it can lead to several health problems. Short-term or acute exposure can cause immediate problems like skin and eye irritation, headaches, dizziness, nausea, and in severe cases, seizures or loss of consciousness.
Long-term, or chronic, exposure can lead to more serious health issues. Researchers have linked pesticide exposure to various health problems, including cancer, problems with our nervous system and hormones, and even kidney disease.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Linking Pesticides and Kidney Problems
In recent years, scientists have started to find connections between pesticides and kidney disease. A study in Sri Lanka found a strange type of kidney disease that wasn't caused by the usual suspects like diabetes or high blood pressure. This disease was mostly found in areas where a lot of pesticides were used.
Another study in Nicaragua, a country in Central America, found a similar connection. People working in agriculture who were exposed to pesticides seemed to have more kidney problems than those who weren't exposed to these chemicals.
A recent study in Texas looked at the role of pesticide exposure in the development of kidney disease in migrants who developed this type of kidney disease. Researchers found that exposure to many agrochemicals, particularly paraquat, was associated with kidney disease.
And it's not just in other countries. Analysis of the National Health and Nutrition Examination Survey (NHANES) data in the United States showed that people who are exposed to pesticide malathion are at a higher risk of developing serious kidney disease.
How Could Pesticides Harm the Kidneys?
The exact process of how pesticides could harm the kidneys isn't fully known. But scientists have a few ideas.
One possibility is that pesticides cause oxidative stress in our cells, including the cells in our kidneys. This cellular stress can lead to damage and cause the cells to function poorly.
Pesticides could also damage DNA, the blueprint for all our body's cells. If the DNA in kidney cells gets damaged, it can lead to kidney disease.
Another theory is that pesticides cause inflammation, which is like a fire inside our body. This internal fire can damage many parts of the body, including the kidneys.
The kidneys act as a filter for our blood, removing waste products and balancing the levels of water and minerals. Because of this role, the kidneys can be exposed to higher levels of harmful substances, including pesticides.
The Way Forward: Protection and Prevention
Knowing that pesticides could lead to kidney problems, it's vital to take steps to protect people from these chemicals. This is particularly important for people who work directly with pesticides, such as farmers and other agricultural workers.
Protecting workers
Safety measures include providing protective clothing, such as gloves and masks, that can reduce direct contact with pesticides. Training programs that educate workers about the safe handling, storage, and disposal of pesticides are also crucial. Furthermore, we can develop and enforce regulations that limit the types and amounts of pesticides used.
Moreover, we should strive to minimize pesticide use wherever possible. There are many different farming techniques that can help achieve this goal. For example, integrated pest management (IPM) is a strategy that aims to control pests in a way that minimizes harm to people and the environment. It involves carefully monitoring crops for pests and only using pesticides as a last resort. Organic farming is another approach that avoids the use of synthetic pesticides altogether.
Protecting yourself
For those who do not work directly with pesticides, minimizing exposure can be more challenging due to their widespread use. However, actions such as washing fruits and vegetables before eating, choosing organic foods, and reducing consumption of processed foods can help reduce the risk.
In the medical field, providers need to be aware of this potential link between pesticides and kidney disease. When they see patients with kidney problems, they should consider whether these patients might have been exposed to pesticides. This awareness can guide their decisions about tests and treatments.
The bottom line
While we still need more research to fully understand the link between pesticides and kidney disease, the evidence so far is a clear warning sign. Pesticides, while helpful for food production, can potentially harm our health, specifically our kidneys. However, knowing about this potential risk is a powerful tool to take steps to protect ourselves and our communities.
The Remarkable Benefits of Omega-3 Fatty Acids in Kidney Disease
Exploring the profound benefits of Omega-3 Fatty Acids in Kidney Disease (CKD) has become a focal point in the healthcare sphere. Derived from fatty fish, flaxseeds, walnuts, and certain algae, these essential nutrients are making waves in the realm of kidney health, demonstrating promising effects for individuals grappling with kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
Anti-Inflammatory Properties of Omega-3 Fatty Acids
Omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been found to exert strong anti-inflammatory properties. Chronic kidney disease is an inflammatory disorder. High levels of inflammatory markers have been reported in patients with CKD. Omega-3 fatty acids (n−3FA) have the capacity to dampen this inflammation, thereby potentially reducing injury to the kidneys and slowing the progression of the disease.
Omega-3 Fatty Acids Balancing Lipid Profiles in Kidney Disease
Another vital benefit of omega-3 fatty acids in kidney disease is their ability to impact lipid profiles positively. A common issue associated with CKD is dyslipidemia, an abnormal amount of lipids (e.g., cholesterol, triglycerides) in the blood. This condition can lead to atherosclerosis, which may further deteriorate kidney function. Studies have shown that omega-3s can improve lipid profiles, reducing levels of harmful lipids and thereby potentially protecting against kidney function decline.
Role of Omega-3 Fatty Acids in Blood Pressure Regulation
Omega-3 fatty acids play a crucial role in blood pressure regulation. Hypertension, or high blood pressure, is both a cause and a consequence of kidney disease. By helping to lower blood pressure, omega-3s may help to mitigate one of the significant risk factors for kidney disease progression.
Significant evidence supports the notion that omega-3 fatty acids can lower blood pressure, especially in individuals with hypertension or high-normal blood pressure levels. To observe a notable reduction in blood pressure, the suggested daily intake of omega-3 fatty acids would likely need to be in the range of 3-4 grams.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Omega-3 Fatty Acids Reducing Proteinuria
Proteinuria, the excessive excretion of protein in the urine, is a prominent symptom of kidney disease. Proteinuria is also a marker of inflammation. It increases the risk of heart disease. Omega-3 fatty acids have demonstrated an ability to decrease proteinuria levels, contributing to improved overall kidney health. Some researchers believe that these effects are due to N-3FA on blood pressure control.
Omega-3 Fatty Acids in IgA nephropathy
IgA nephropathy, also known as Berger's disease, is a kidney disorder that arises when IgA - a protein meant to protect the body from infections - settles in the kidneys, causing inflammation that gradually hinders kidney function.
Research suggests that omega-3 fatty acids can play a role in managing this condition. The anti-inflammatory properties of omega-3 fatty acids may help reduce the inflammation associated with IgA nephropathy, potentially slowing its progression. Indeed, several studies have shown that N-3FA can slow down the progression of IgA nephropathy at various doses.
Omega-3 Fatty Acids in Cardiovascular Health Management
Omega-3s can help manage complications of kidney disease, such as cardiovascular disease. Cardiovascular disease is the leading cause of death among individuals with kidney disease. N-3FA has well-documented heart health benefits in patients with CKD. This includes reducing inflammation, improving lipid profiles, and lowering blood pressure. Therefore, they may reduce the risk of heart disease in individuals with CKD.
Recommended Dosage of Omega-3 Fatty Acids in Kidney Disease
Omega-3 supplements typically contain EPA and DHA, with dosages often specified by the amounts of these two ingredients. While many studies outline doses in terms of the ingredient used, others express them as grams of fish oil per day. EPA, renowned for its superior anti-inflammatory properties, is particularly beneficial. A daily intake of 2.5-3 grams of EPA has been demonstrated to significantly slow the progression of chronic kidney disease.
Finally, it is essential to ensure that you get a high-quality omega-3 supplement such as those found at Fullscript. When purchasing omega-3 products, opt for those which are certified for purity or bear a third-party seal. This guide can help you identify quality supplements.
Omega-3s also tend to spoil over time, losing their potency and possibly becoming harmful when rancid. They emit an unpleasant odor when spoiled. Therefore, it's crucial to inspect the product's date and smell it for freshness. Be sure to store your N-3FA supplement in the refrigerator, take it with meals, and avoid taking it with soda or coffee.
Integrating Omega-3 Fatty Acids into Comprehensive Kidney Disease Care
A diet rich in Omega-3 fatty acids has been shown to slow down the progression of CKD. Omega-3 fatty acids should not be considered a standalone treatment for kidney disease but an essential part of a comprehensive dietary and lifestyle approach. Their potential benefits in kidney disease management make them a vital part of the diet for kidney health.
The bottom line
Omega-3 fatty acids in kidney disease have shown potential as a valuable dietary intervention, improving overall kidney health and slowing disease progression when used as part of a comprehensive care plan.
Three Common Mistakes Kidney Patients Make When Taking Vitamin D Supplements
Vitamin D holds a unique and vital position in the vast array of nutrients essential to our well-being. Its influence spans various physiological functions, from bone health to immune system strength. For those living with kidney disease, maintaining an adequate level of vitamin D becomes even more crucial as it helps in managing bone health and mineral balance. Still, it's not as simple as just taking a supplement. This blog will discuss mistakes kidney patients make when taking vitamin D supplements.
Patients with kidney disease face a unique set of considerations when taking vitamin D supplements. To guide you through this process, we've identified three common mistakes that many kidney disease patients make.
By Majd Isreb, MD, FACP, FASN, IFMCP
Vitamin D mistake #1. Not Checking Vitamin D Levels
It's crucial for anyone considering vitamin D supplements to check their vitamin D levels first, but it's particularly essential for patients with kidney disease. Some studies showed that as many as 80% of patients with chronic kidney disease have vitamin D deficiency. Due to changes in kidney function, these individuals often struggle with regulating their vitamin D levels, making regular checks a necessary part of their health routine.
Vitamin D testing typically involves analyzing a blood sample for the concentration of 25(OH)D, the best marker for assessing vitamin D status in the body. This test provides vital information to ensure patients are getting enough vitamin D. Enough to support their bone health and immune function. But not too much to increase the risk of hypercalcemia, a condition characterized by too much calcium in the blood. Hypercalcemia can worsen kidney problems.
In fact, vitamin D is used in large amounts as a rodenticide as it causes hypercalcemia and death in rodents.
Conventional providers may aim for a target level of 30 ng/mL. Some experts even advocate for lower levels. We believe that vitamin D levels should be checked twice a year in kidney patients. The target level for vitamin D in kidney patients should be 50-60 ng/mL as long as hypercalcemia is avoided.
Vitamin D mistake #2. Not taking Vitamin K2 with it
The interaction between vitamin D and vitamin K2 is crucial for maintaining a healthy calcium balance in the body. While vitamin D aids calcium absorption in the gut, vitamin K2 helps to direct this calcium to the bones, where it's needed, and away from arteries and other soft tissues, where it can cause harm.
For kidney disease patients, this relationship becomes even more critical. Vitamin K deficiency is common in kidney patients. Studies have shown that vitamin K2 can help to lower the risk of vascular calcification, a common problem in those with kidney disease. However, keep in mind that some anticoagulant medications can interact with vitamin K2. If you're on these medications, it's essential to consult with your healthcare provider before beginning a regimen of vitamin K2.
Vitamin D mistake #3. Not Taking Magnesium with Vitamin D
The last common mistake kidney disease patients make is not taking magnesium with their vitamin D supplement. Magnesium is involved in the conversion of vitamin D into its active form, which the body can use. A deficiency in magnesium can hinder this process, potentially making vitamin D supplements less effective.
Furthermore, supplementing vitamin D leads to increase utilization of magnesium. This may lead to magnesium deficiency. Therefore, it becomes crucial to check and supplement magnesium in kidney patients taking vitamin D.
However, kidney disease patients need to approach magnesium supplementation with caution. Advanced kidney disease can lead to an excess of magnesium in the body, a condition known as hypermagnesemia because the kidneys are less able to remove this mineral.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Bonus Mistake: Not Prioritizing Supplement Quality
As is the case with any supplement, the quality of the vitamin D supplement you choose to take significantly influences its effectiveness and your health. All supplements are not created equal, and with a market inundated with various brands and types of vitamin D supplements, picking the right one can be challenging.
Firstly, it's crucial to understand that the supplement industry is loosely regulated. This means that the onus of ensuring product safety and efficacy often falls on the manufacturers. Unfortunately, not all companies adhere to stringent manufacturing practices, leading to products that may contain contaminants or do not contain the stated amount of the vitamin.
For patients with kidney disease, this could potentially pose serious health risks. Low-quality supplements might contain harmful additives or contaminants that could worsen kidney damage. Alternatively, they may not provide the stated amount of vitamin D, leading to suboptimal levels despite supplementation.
To avoid this mistake, always opt for supplements from reputable brands that adhere to Good Manufacturing Practices (GMPs) and have their products independently tested for purity and potency. Look for brands that are transparent about their sourcing and manufacturing processes. When in doubt, seek guidance from your Integrative healthcare provider to help you select a high-quality vitamin D supplement that is suitable for your specific needs.
The bottom line
Although vitamin D supplements can play a vital role in managing kidney disease, they need to be taken correctly to optimize their benefits and prevent possible complications. Regularly checking your vitamin D levels, considering the role of vitamin K2, and discussing magnesium supplementation with your Integrative healthcare provider will ensure that you're making the most out of your vitamin D supplements. As always, personal healthcare decisions should be made in consultation with a trusted medical professional who understands your unique health situation.
Effects of EBV infection on the kidneys
Epstein-Barr virus (EBV) is a human herpes virus that infects approximately 95% of the world's population. It primarily infects B-lymphocytes and can cause a range of clinical manifestations, including infectious mononucleosis, chronic active EBV infection, and malignancies such as Burkitt's lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma. In addition to these well-known associations, EBV infection has also been linked to kidney diseases. In this blog, we will discuss the effects of EBV infection on the kidneys and the mechanisms involved.
By Majd Isreb, MD, FACP, FASN, IFMCP
Effects of EBV infection on the kidneys
EBV, also known as human herpesvirus 4, infects up to 95% of the population, primarily in childhood, then establishes a lifelong latent infection in memory B cells. EBV infection has been recently implicated in the development of several autoimmune diseases, including multiple sclerosis, lupus, rheumatoid arthritis, Sjögren's syndrome, and autoimmune thyroid diseases.
In the kidneys, Epstein-Barr virus (EBV) infection has been associated with several kidney diseases, including acute interstitial nephritis (AIN), membranous nephropathy, minimal change disease (MCD), and more rarely, focal segmental glomerulosclerosis (FSGS).
EBV and acute interstitial nephritis (AIN)
Acute interstitial nephritis (AIN) is an inflammatory disease that affects the interstitial tissue of the kidneys, which surrounds the tubules. AIN is a rare complication of EBV infection but has been reported in some cases. The exact mechanisms by which EBV infection causes AIN are not fully understood. Still, it is thought that the immune response to EBV infection may trigger an inflammatory response in the kidneys, leading to tissue damage and inflammation.
EBV and IgA nephropathy
In addition to AIN, EBV infection has also been implicated in developing other renal diseases, such as IgA nephropathy and membranous nephropathy. For example, in IgA nephropathy patients, EBV infection has been found to trigger the production of aberrantly glycosylated IgA1. This was not the case in healthy controls. This supports the hypothesis that these patients have a predisposition (genetic or otherwise) to form these aberrantly glycosylated IgA1.
In essence, IgA nephropathy patients tend to form abnormal IgAs, then a trigger such as EBV can induce the mass formation of these abnormal IgAs. These abnormal IgAs then cause an immune response in which the body forms antibodies against these abnormal proteins. The resultant immune complexes then deposit in the glomeruli, leading to glomerulonephritis. Furthermore, EBV has been thought to be the reason behind the racial distribution of IgA nephropathy.
EBV and membranous nephropathy
Membranous nephropathy is a glomerular disease affecting the kidneys' filtering units, the glomeruli. EBV infection has been associated with the development of membranous nephropathy in some cases. In membranous nephropathy, EBV infection is thought to cause the formation of antibodies that cross-react with a major podocyte protein causing it to be lost in the urine. This will cause significant dysfunction in the glomerular wall filtration system (slit diaphragm) and loss of a massive amount of protein in the urine.
EBV in minimal change disease and FSGS
Minimal change disease (MCD) is another glomerular disease that affects the podocytes in the glomeruli. In some cases, EBV infection has been linked to the development of MCD, although the exact mechanisms are not fully understood. It is thought that EBV infection may trigger an autoimmune response that damages the podocytes and leads to the development of MCD.
Primary Focal segmental glomerulosclerosis (FSGS) is another autoimmune kidney disease. EBV infection has rarely been implicated in the development of FSGS, but the evidence linking EBV to FSGS is limited. The exact mechanisms by which EBV infection may lead to the development of FSGS are not fully understood.
EBV-associated nephropathy
EBV-associated nephropathy (EBVAN) is a rare but potentially life-threatening complication of EBV infection that primarily affects immunocompromised individuals, such as transplant recipients and patients with AIDS. EBVAN is characterized by the presence of large atypical lymphoid cells in the renal tubules and interstitium, which can cause tubulointerstitial nephritis, interstitial fibrosis, and, eventually, kidney failure.
The exact mechanisms of EBVAN pathogenesis have yet to be fully understood. Still, it is thought to involve direct infection of renal epithelial cells by the virus and the subsequent activation of immune responses against infected cells.
Mechanism of EBV-associated kidney diseases
If EBV infects 95% of the population, why do some people develop autoimmune diseases related to EBV while others do not?
While it is true that up to 95% of the population has been infected with Epstein-Barr virus (EBV) at some point in their lives, not everyone who is infected with EBV will go on to develop autoimmune diseases related to EBV. Also, not every case of the autoimmune kidney disease mentioned above is associated with EBV.
The development of autoimmune diseases is a complex process involving genetic, environmental, and immunological factors. One proposed mechanism for developing autoimmune diseases related to EBV is molecular mimicry. Due to structural similarities between EBV antigens and self-antigens, the immune system mistakenly identifies self-antigens as foreign antigens. This can lead to an autoimmune response against self-tissues, resulting in tissue damage and inflammation.
Another proposed mechanism is that EBV infection may dysregulate the immune system, leading to a breakdown in self-tolerance and the development of autoimmune diseases. For example, EBV infection has been shown to activate B and T cells, producing autoantibodies and activating autoreactive T cells.
However, the development of autoimmune diseases is likely to be multifactorial and involve a combination of genetic and environmental factors. While EBV infection may contribute to the development of autoimmune diseases in some individuals, other factors, such as genetic predisposition, environmental triggers, and other infections, may also play a role.
Testing for EBV
Epstein-Barr virus (EBV) infection can be diagnosed using various laboratory tests, including serological tests, molecular tests, and viral culture.
Serological tests detect antibodies to EBV in the blood. These tests can determine whether an individual has been infected with EBV in the past or is currently infected. Two main types of antibodies can be detected: IgM and IgG. IgM antibodies are produced early in the course of infection, while IgG antibodies are produced later and persist for life. Therefore, a positive IgM test indicates acute infection, while a positive IgG test indicates previous or current infection.
Antibodies to the viral capsid antigen (VCA) appear early in EBV infection and peak between 2-4 weeks after the appearance of infectious mononucleosis. In comparison, early antigen IgG antibodies (EA-D IgG) are usually associated with the acute stage of EBV infection. Finally, EB nuclear antigen IgG antibodies are conventionally thought to indicate prior exposure to EBV. Therefore, we recommend using a panel of all these antibodies when testing a patient for latent or active EBV. The following table will help you interpret these results:
| VCA-IgM | VCA-IgG | EA-D, IgG | EBNA IgG | Likely interpretation |
| Negative | Negative | Negative | Negative | No EBV infection |
| Positive | Positive | Negative | Negative | Early primary infection |
| Negative or positive | Positive | Positive | Negative | Active infection |
| Negative | Positive | Negative | Positive | Prior infection |
| Negative | Positive | Positive | Positive | May indicate reactivation |
Molecular tests detect the presence of EBV DNA in the blood or other body fluids, such as saliva or cerebrospinal fluid. Polymerase chain reaction (PCR) is the most commonly used molecular test for the detection of EBV DNA. However, unfortunately, PCR can detect latent EBV in as low as 54% and as high as 94% of seropositive individuals, according to the tests above. In addition, it has poor sensitivity and specificity even in primary EBV infection.
Viral culture involves the isolation and growth of the virus from a sample, such as blood or saliva. However, viral culture is rarely used to diagnose EBV infection due to its low sensitivity and the time required for the virus to grow.
How to treat EBV or chronic EBV infections
There is no specific treatment for EBV infection or chronic latent EBV infections.
Treatment for chronic latent EBV infections may involve a combination of antiviral medications, immunomodulatory drugs, and supportive care. However, there is no consensus on the optimal treatment approach, and the treatment plan should be individualized based on the patient's symptoms and clinical presentation.
Antiviral medications, such as acyclovir, valganciclovir, and ganciclovir, may suppress EBV replication and reduce the viral load. However, these medications have limited efficacy against EBV.
Immunomodulatory drugs, such as corticosteroids, rituximab, and interferon, may modulate the immune response and reduce inflammation. These drugs have been shown to be effective in some patients with chronic EBV infections, but their use is associated with potential side effects and should be carefully monitored.
Supportive care, such as rest, hydration, and a healthy diet, can also help to manage symptoms and promote recovery in patients with chronic EBV infections. However, it is essential to note that managing chronic latent EBV infections is complex and requires a multidisciplinary approach.
Join us to end the kidney disease epidemic
Is there a natural treatment for EBV?
Several natural treatments may help manage EBV infection symptoms, although limited scientific evidence supports their effectiveness. These include:
- Rest and stress reduction: Rest and stress reduction can help to boost the immune system and promote healing. Getting adequate sleep, practicing relaxation techniques, such as meditation or yoga, and avoiding stressors can be helpful.
- Balanced diet: Eating a balanced diet rich in fruits, vegetables, whole grains, and lean proteins can help to support the immune system and reduce inflammation.
- Herbal remedies: Certain herbs, such as echinacea, elderberry, and astragalus, have been traditionally used to boost the immune system and may help manage EBV infection.
- Probiotics: Probiotics, which are beneficial bacteria that live in the gut, may help to support the immune system and reduce inflammation. Consuming probiotic-rich foods, such as yogurt or kefir, or a probiotic supplement may be helpful.
- Vitamin and mineral supplements: Certain vitamins and minerals, such as vitamin C, vitamin D, zinc, and selenium, are essential for immune function and may help manage EBV infection. However, consulting with an Integrative healthcare provider before taking any supplements is important.
It is important to note that natural treatments should not be used as a substitute for a comprehensive integrative approach. Additionally, it is essential to consult with an integrative healthcare provider before starting any natural treatment, as some natural treatments may interact with medications or have potential side effects.
The bottom line
EBV is a highly prevalent virus affecting approximately 95% of the world's population. It primarily infects B-lymphocytes, making them immortal. EBV has been linked to many autoimmune diseases. For example, it has been linked to autoimmune kidney diseases such as IgA nephropathy, membranous nephropathy, minimal change disease, and FSGS. Testing these patients for latent EBV infection is, therefore, important.
May Research and News 2023
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney May Research and News.
May Research and News
Lifestyle interventions do indeed work
Poor dietary patterns and low physical activity levels are important lifestyle-related factors that contribute to chronic kidney disease (CKD) and its progression.
In a systematic review and meta-analysis published in Kidney Medicine, researchers evaluated the impact of diet, exercise, and other lifestyle-related interventions on risk factors for and progression of chronic kidney disease (CKD) and quality of life.
The analysis included 68 randomized controlled trials in individuals with CKD.
Twenty-four studies were dietary interventions, 23 were exercise, 9 were behavioral, 1 a hydration intervention, and 11 involved multiple components.
Lifestyle interventions resulted in significant improvements in:
1. Creatinine
2. 24-hour albuminuria
3. Systolic and diastolic blood pressure
4. Body weight
5. Quality of life (mostly sleep, fatigue, and pain)
Lifestyle interventions did not result in significant changes in the estimated glomerular filtration rate (eGFR)!
You would wonder how the study shows improvement in creatinine with lifestyle interventions but not in eGFR, which is calculated from creatinine. The authors explained that the changes in creatinine "were not clinically significant."
Lifestyle interventions resulted in significant improvements in creatinine (-0.43mg/dL, 95% CI: -0.74, -0.11, p=0.008). In our minds, a 0.4 drop in creatinine is significant, and it beets worsening creatinine at any time.
Finally, the meta-analysis demonstrated that exercise interventions resulted in the greatest improvements in eGFR and creatinine. While the greatest effects of dietary interventions were albuminuria and systolic blood pressure.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The four-hit hypothesis in IgA Nephropathy
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common primary glomerulonephritis worldwide.
The ongoing theory about the root cause of IgAN is the “four-hit hypothesis.” This hypothesis suggests that four hits cause the development of IgAN:
1. Increased circulating levels of an abnormal IgA1 (gd-IgA1)
2. The production of autoantibodies against this abnormal IgA1
3. The formation of immune complexes with anti-gd-IgA1 antibodies
4. The deposition of these immune complexes in the glomerular mesangium leads to kidney
The site of gd-IgA1 production is presumed to be the Peyer's patches of the gut and mesenteric lymph nodes.
No one knows why patients start producing this abnormal IgA1. However, an interplay between genetics and the environment may play a role.
We believe that environmental factors (dysbiosis, virus, infection, etc.) may trigger the formation of abnormal IgA1 in genetically predisposed patients.
This review article provides very insightful details about the role of innate and adaptive immune mechanisms involved in IgAN for those who like to dig deeper. It is a free-access article.
Speaking of IgA nephropathy
A recent study in CJASN looked at the long-term outcomes for patients with IgAN. The study included 2,299 adults and 140 children from the UK National Registry of Rare Kidney Diseases.
50% of patients reached kidney failure or died over a median follow-up of 5.9 years.
The median kidney survival was 11.4 years, with an average age at kidney failure or death was 48 years.
Most patients progressed to kidney failure within 10-15 years.
Time-averaged proteinuria was significantly associated with worse kidney survival and rapid kidney function loss.
Why is this important? Besides knowing the natural progression of IgAN, many pharmaceutical companies are developing drugs for managing IgAN. They are targeting proteinuria as a surrogate marker for the progression of IgAN.
You should know that this study is sponsored by Travere Therapeutics, the maker of the new IgAN drug, Sparsentan. One of the authors works in Travere Therapeutics, and others are consultants for it.
Overweight moms are more likely to have children with congenital abnormalities in their kidneys and urinary tract
In a study of 4,619 newborns enrolled during 2012–2020 from a hospital in Taiwan, maternal risk factors before and during pregnancy were compared in children with and without congenital anomalies of the kidney and urinary tract (CAKUT).
In total, 73 (1.6%) cases of CAKUT in offspring were identified.
Maternal overweight before pregnancy (BMI ≥ 24 kg/m2) was an independent risk factor for CAKUT in offspring.
There were no observed associations of pregestational diabetes and gestational diabetes with CAKUT in offspring were observed.
“Maternal obesity before pregnancy is associated with CAKUT in offspring and should be addressed to ensure better outcomes,” note the authors of the Pediatric Nephrology study.
This study highlights the role of epigenetics in kidney health and disease.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
Understanding Ferroptosis: A Closer Look at Cellular Iron Imbalance and Glutathione Regulation
Programmed cell death is an important biological process. It removes damaged or unnecessary cells in a controlled fashion. Ferroptosis is one of these processes. It is gaining momentum as an important process in several diseases. These include cancer, neurodegenerative disorders, and kidney disease. In this blog, we will explore the fascinating world of ferroptosis. We will focus on the role of cellular iron imbalance and the role of glutathione. We will also discuss the role of ferroptosis in kidney disease.
By Majd Isreb, MD, FACP, FASN, IFMCP
Ferroptosis: The Iron-Dependent Cell Death
Ferroptosis is a unique type of programmed cell death. Here, reactive oxygen species (ROS) and lipid peroxides accumulate in the cell. This ultimately leads to iron-dependent damage to cell membranes. The latter leads to cell death.
The name "ferroptosis" comes from the Latin word "Ferrum" (iron) and the Greek word "ptosis" (falling). The name highlights the critical role of iron in this process. Unlike apoptosis or necrosis, ferroptosis involves an imbalance in cellular iron metabolism.
Ferroptosis vs. apoptosis
Ferroptosis and apoptosis are both types of programmed cell death. Yet, they have different characteristics and mechanisms.
Apoptosis is a regulated process that occurs in response to various signals. These include DNA damage or a lack of nutrients, for example. During apoptosis, the cell undergoes a series of molecular changes. These lead to the dismantling of the cell into small fragments (apoptotic bodies). These are then removed by immune cells without causing inflammation.
In contrast, in ferroptosis, there is a buildup of specific lipids. Phospholipids contain polyunsaturated fatty acids that make them vulnerable to oxidation. Excess iron in the cell reacts with hydrogen peroxide (H2O2), forming reactive oxygen species (ROS). ROS, in turn, reacts with the phospholipids of the cell membrane. This forms lipid peroxides which end up accumulating in the cell. The process ends up damaging the cell membrane in an iron-dependent manner. It leads to a loss of membrane integrity and, ultimately, cell death. Ferroptosis, unlike apoptosis, does not release apoptotic bodies but can induce inflammation.
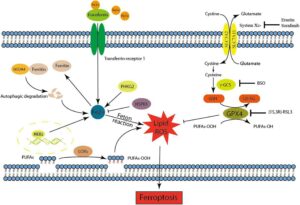
Cellular Iron Imbalance and Ferroptosis
Cellular iron imbalance is a key trigger of ferroptosis. Iron, an essential element for various cellular processes, can be beneficial and detrimental. Excess iron can react with oxygen generating ROS. ROS, in turn, causes oxidative damage to lipids, proteins, and DNA. Ferroptosis occurs when there is a disruption in cellular iron metabolism. This disruption leads to iron accumulation and then lipid peroxidation.
Studies have shown that iron chelators block ferroptotic cell death. In addition, various iron transporters are involved in iron metabolism. Mutations in iron transporter genes and external stressors can contribute to iron imbalance.
The Role of Glutathione in Ferroptosis
Glutathione is a tripeptide molecule composed of glutamate, cysteine, and glycine. It is a potent cellular antioxidant that scavenges and neutralizes ROS. This makes it a master antioxidant because it protects cells from oxidative stress. In ferroptosis, there is a disruption in the balance between glutathione and ROS. Depletion of glutathione leads to an accumulation of lipid peroxides. These, in turn, disrupt cell membrane integrity and promote ferroptosis, as we saw.
Several factors can cause the depletion of glutathione in the cells. The enzyme glutathione peroxidase 4 (GPX4) is a regulator of ferroptosis. It depends on the availability of glutathione to function. Lower glutathione levels decrease the activity of GPX4. This, again, causes the accumulation of lipid peroxide and ferroptosis.
Similarly, cystine is an oxidized form of the amino acid cysteine. It is a precursor for glutathione synthesis and can regulate ferroptosis. There is a specific system that transports cystine into cells. This cystine/glutamate antiporter system Xc- plays critical for maintaining intracellular glutathione levels. Inhibition of this system can lead to cystine depletion. This, in turn, causes glutathione depletion and ferroptosis.
Inhibition of Cystine/Glutamate Antiporter System
The cystine/glutamate antiporter system Xc- regulates intracellular glutathione levels. Various factors can inhibit this antiporter. Compounds like erastin and sulfasalazine can inhibit it. These compounds can cause glutathione depletion and trigger ferroptosis. Oxidative stress, amino acid imbalances, and certain chemotherapeutic drugs can also disrupt it.
Kidney Disease Implications
Ferroptosis plays a role in various kidney diseases. These include acute kidney injury (AKI), chronic kidney disease (CKD), and kidney cancer. In AKI, factors such as ischemia-reperfusion injury and drug nephrotoxicity can trigger ferroptosis. This causes renal cell death and kidney damage.
Iron metabolism is dysregulated in CKD. Glutathione balance is also disturbed. Both of these factors contribute to the progression of CKD. There is an increasing body of evidence indicating that ferroptosis is involved in the progression of CKD.
This highlights the crucial role of oxidative stress in kidney injury and disease. It also emphasizes the need to support glutathione in kidney patients. Iron metabolism and regulation may be another target to slow the progression of CKD.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The bottom line
Ferroptosis is an iron-dependent form of programmed cell death. It has emerged as a significant player in kidney diseases. Disturbances in cellular iron metabolism and glutathione levels contribute to oxidative stress. This causes lipid peroxidation of cell membranes and then death in renal cells. Ferroptosis is a link between iron metabolism, oxidative stress, glutathione, and kidney injury. It plays an important role in the progression of kidney disease.
Air pollution and kidney health
Air pollution is a major public health concern that affects millions of people around the world. It is associated with various adverse health outcomes, including respiratory diseases, cardiovascular diseases, and cancer. However, recent research has also linked air pollution to kidney diseases, including glomerulonephritis and chronic kidney disease (CKD). In this blog, we will discuss the relationship between air pollution and kidney health.
By Majd Isreb, MD, FACP, FASN, IFMCP
Air pollution is defined as the presence of harmful substances or contaminants in the air that negatively affect human health, the environment, or the quality of life. These substances can be both natural (e.g., dust, pollen) and human-made (e.g., emissions from vehicles, industrial processes, and power generation). According to a new report from the American Lung Association, nearly 1 in 5 Americans live in communities with harmful air quality.
Air pollution and kidney health
Particulate matter (PM)
When it comes to health, air pollution is typically measured based on the concentration of specific pollutants in the air. One of these measures is particulate matter (PM). These are tiny particles suspended in the air, typically measured in two size categories - PM10 (particles with a diameter of 10 micrometers or smaller) and PM2.5 (particles with a diameter of 2.5 micrometers or smaller).
PM2.5 is of particular concern as these tiny particles can penetrate deep into the lungs and cause respiratory and cardiovascular issues. It is what I will focus on in this blog.
Air pollution and glomerulonephrites
Glomerulonephritis is a type of kidney disease that affects the glomeruli, the small blood vessels in the kidneys that filter waste and excess fluids from the blood. Various factors, including infections, autoimmune disorders, and exposure to toxins, can cause it. Air pollution has been identified as a potential risk factor for glomerulonephritis, particularly in urban areas where levels of air pollution are high.
One study published in the Journal of the American Society of Nephrology found that exposure to PM2.5 was associated with an increased risk of developing membranous nephropathy in China. Each 10 μg/m3 increase in PM2.5 concentration was associated with 14% higher odds for membranous nephropathy.
Another study found that PM2.5 is an independent risk factor for the progression of kidney disease in patients with IgA nephropathy. Air pollution has also been implicated in the epigenetic changes that promote the development of lupus.
Join us to end the kidney disease epidemic
Air pollution and CKD
Chronic kidney disease (CKD) is a progressive disease in which the kidneys gradually lose their ability to filter waste and excess fluids from the blood. It is a major global health problem, affecting an estimated 10% of the adult population worldwide. Air pollution has also been linked to an increased risk of CKD.
One study published in the Journal of the American Society of Nephrology found that exposure to high levels of PM2.5 was associated with a higher risk of CKD in China. A recent study in Minnesota found that higher PM2.5 was also associated with an increased incidence of CKD.
The largest to date study on the link between PM2.5 and CKD linked the Environmental Protection Agency and the Department of Veterans Affairs databases. The study, which involved more than 2 million adults in the United States, found that those who were exposed to higher levels of PM2.5 were more likely to develop CKD, have a faster decline in eGFR, and need dialysis than those who were not exposed to high levels of air pollution.
How does air pollution damage the kidneys?
The exact mechanisms by which air pollution causes kidney diseases are not fully understood. However, it is believed that air pollution may damage the kidneys by causing inflammation, oxidative stress, and endothelial dysfunction. Air pollution can also affect the kidneys indirectly by promoting hypertension (high blood pressure), insulin resistance, and diabetes.
Preventing the effects of air pollution on the kidneys
Reducing air pollution is, therefore, an important strategy for preventing kidney diseases. There are big societal measures that can be used to achieve that goal. These include reducing emissions from vehicles and industry, promoting clean energy sources, and increasing access to public transportation.
However, on the individual level, there are many strategies that can be used to decrease exposure. This reference contains detailed recommendations. I summarized them here:
- Verify the air quality in your area. You can check it in the US on this website.
- Avoid outdoor activity when and where the air pollutant levels are higher.
- Avoid high traffic and industrialized areas as much as possible.
- Maintaining good indoor air quality by using air filters and air purifiers.
- Use personal protective equipment when necessary.
- A diet high in antioxidants can also be protective.
The bottom line
Air pollution is a significant risk factor for kidney diseases, including glomerulonephritis and CKD. It is, therefore, important for individuals, communities, and policymakers to take action to reduce exposure to air pollution and promote clean, healthy environments.
The Thyroid-Kidney Connection
The thyroid and kidneys are two vital organs in our body, and they play a crucial role in maintaining our overall health. Recent research has highlighted the link between thyroid and kidney diseases, showing that thyroid dysfunction can lead to kidney problems and vice versa. In this blog, we will discuss the thyroid-kidney connection.
By Majd Isreb, MD, FACP, FASN, IFMCP
The thyroid gland function
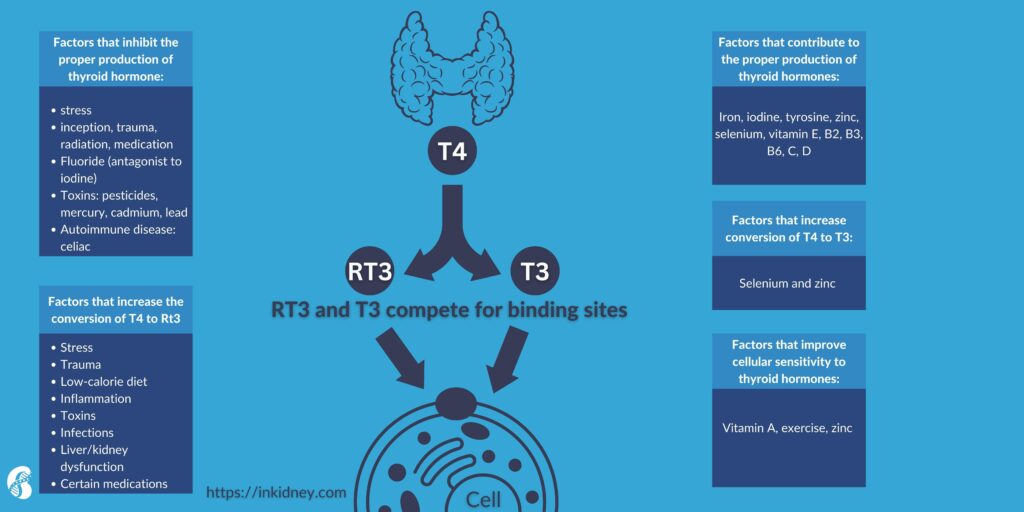
Before we discuss the thyroid-kidney connection, it is helpful to discuss the function of the thyroid gland briefly. The thyroid gland releases triiodothyronine (T3) and thyroxine (T4). These hormones play an important role in the regulation of your weight, energy levels, internal temperature, skin, hair, nail growth, and metabolism.
The pituitary gland produces a hormone called thyroid stimulating hormone (TSH) which propels the thyroid to produce T3 and T4. There is something called a feedback loop. So, when the levels of T3 and T4 increase, they prevent the release of TSH. When T3 and T4 levels drop, the feedback loop starts again. This system allows your body to maintain a constant level of thyroid hormones.
T4 is converted into T3, which is the active form of thyroid hormone. About one-third of T4, though, gets converted to something called reverse T3 (rT3). RT3 is not believed to be not an active hormone. Researchers believe the body produces rT3 in times of severe illness or starvation as a mechanism for preserving energy.
T4 and T3 circulate in the blood bound to a protein called thyroid binding globulin (TBG). The free forms of T3 and T4 are the active forms of thyroid hormone. Free T3 enters the target cells and the nucleus to regulate various genes.
Testing thyroid function
By this quick review, you can realize that thyroid hormones are complex and testing them should be more involved than the conventional medicine TSH screening. A comprehensive testing approach to the thyroid gland function should include assessing TSH, free T3, free T4, RT3, total T3, Total T3/RT3 ratio, FT3/FT4 ratio, thyroid Antibodies (anti-thyroid peroxidase antibodies, and anti-Antithyroglobulin antibodies).
The thyroid-kidney connection
The thyroid hormones play an important role in the growth and development of the kidneys. Thyroid function also influences water and electrolyte balance in different compartments of the body. But more importantly, the thyroid affects kidney function itself. On the other hand, the kidneys play an important role in the metabolism and elimination of thyroid hormones.
The effect of thyroid on kidney function
Studies have shown that thyroid deficiency (hypothyroidism) is associated with a decrease in glomerular filtration rate (GFR) and an increase in serum creatinine. Thyroid hormones were found to increase the blood flow to the kidneys, and a deficiency in thyroid hormones causes a decrease in the blood flow, which decreases GFR.
Thyroid excess (hyperthyroidism) is associated with the opposite effects in animal models.
Most of these changes are reversible with appropriate thyroid therapy. However, some researchers found that functional deficiency in thyroid hormones can also be associated with increased protein in the urine. This may indicate that hypothyroidism can lead to true intrinsic kidney disease and glomerulopathy.
The thyroid can also affect the kidneys indirectly by changing cardiac output and peripheral resistance. It also affects the production of insulin-like growth factor type 1 (IGF-1). Thyroid deficiency decreases IGF-1, which can increase insulin resistance and worsen kidney disease.
It is crucial to test for thyroid dysfunction in patients with kidney disease because correcting subclinical hypothyroidism has been found to preserve renal function and improve renal outcomes.
Thyroid disease and glomerulonephrites
Glomerulonephrites (GN) are autoimmune kidney diseases. Both thyroid deficiency and excess were found to be associated with GN. Thyroid dysfunction was found in membranous nephropathy, IgA nephropathy, membranoproliferative glomerulonephritis, and minimal change nephropathy.
This association is likely autoimmune in nature. Both thyroid diseases and glomerulonephrites are associated with circulating autoimmune elements called immunocomplexes. In fact, studies have shown that 26% of patients with thyroid disease have circulating immunocomplexes. This percentage increased to up to 55% in patients with autoimmune thyroid diseases such as Hashimoto’s thyroiditis. The presence of thyroid peroxidase antibodies correlates with the presence of these complexes.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The thyroid and tubular function
The tubules are responsible for the second stage in the process of elimination by the kidneys. Tubulointerstitial nephritis has been seen in patients with thyroid dysfunction. Thyroid excess has been especially seen in patients with tubulointerstitial nephritis and uveitis syndrome (TINU). Studies have shown that antibodies against modified C-reactive protein (mCRP) are possibly implicated in this syndrome and may also be the cause of the associated hyperthyroidism.
Tubulointerstitial nephritis and hyperthyroidism were reported together in patients being treated with rifampicin.
The thyroid and acute kidney injury
Besides the reversible changes in GFR mentioned above, thyroid deficiency has been linked to muscle breakdown (rhabdomyolysis), leading to kidney injury. This has been reported in patients who were treated with statins and patients who were not.
The thyroid effects on electrolytes
Thyroid hormones can cause affect the way the kidney handles water, sodium, potassium, and calcium. The following electrolyte abnormalities have been seen in patients with thyroid disorders:
- Hyponatremia (low serum sodium level) in hypothyroidism.
- Hypercalcemia (high serum calcium level) has been reported in hypothyroidism and hyperthyroidism.
- Hypokalemia (low serum potassium) and hyperkalemia (high serum potassium) were reported in hyperthyroidism.
The effect of kidney disease on the thyroid
The kidneys play an important role in the metabolism and clearance of thyroid hormones. CKD has been found to affect the hypothalamus-pituitary-thyroid axis. TSH can be elevated in CKD. CKD can also alter TSH circadian rhythm. Free and total T3 and T4 concentrations can be low in CKD.
More importantly, the peripheral conversion of T4 to T3 is impaired in CKD. The presence of metabolic acidosis (increased blood acidity), which is common in CKD, can make that worse. Total rT3 can be increased in CKD due to decreased clearance. rT3 can compete with T3 on the cellular level leading to worsening symptoms of thyroid deficiency in CKD.
Studies have shown that the rate of subclinical hypothyroidism increases as GFR decreases. Hypothyroidism is noted in about 18% of patients with eGFR of less than 60 ml/min/1.73 m2. Some researchers believe this is due to a decrease in the clearance of iodine, causing increased serum iodine levels and decreased thyroid hormone production.
Finally, nephrotic syndrome describes kidney diseases that are associated with massive loss of protein in the urine. This can lead to the loss of thyroid-binding globulin in the urine. It can lead to a decrease in serum total T4 and T3. However, free T4 and T3 remain normal, indicating that a normal thyroid can compensate in the case of nephrotic syndrome.
However, patients who have low thyroid reserve can develop overt hypothyroidism if they have nephrotic syndrome. Nephrotic syndrome also increases the need for higher doses of thyroid hormone therapy in patients with hypothyroidism.
The bottom line
There are significant interactions between the thyroid and the kidneys. Thyroid diseases can affect kidney function and are associated with different kidney diseases. Kidney diseases can also lead to various thyroid dysfunction. It is crucial to perform comprehensive testing of the thyroid in patients with kidney disease.
Fluoride and Kidney Health: What You Need to Know
Fluoride is a naturally occurring mineral that's added to many public water systems to prevent tooth decay. While fluoride is generally considered safe at low levels, there's been growing concern about the potential health risks associated with long-term exposure to high levels of fluoride, particularly in relation to kidney health. In this blog, we will focus on fluoride and kidney health.
Floride and Kidney Health
By Majd Isreb, MD, FACP, FASN, IFMCP
What is Fluoride?
Fluoride is a naturally occurring mineral found in soil, water, and many foods. It's also added to some public water systems, toothpaste, and other oral care products to prevent tooth decay. The amount of fluoride in public water systems is typically regulated to prevent excessive exposure.
How Does Fluoride Affect Kidney Health?
There has been growing concern about the potential health risks associated with long-term exposure to high levels of fluoride, particularly in relation to kidney health. So let’s explore them.
Accumulation in the kidneys
The pineal gland (producer of melatonin) and the kidney are exposed to higher concentrations of fluoride than all other organs. Therefore, exposure to higher concentrations of fluoride could contribute to kidney damage, ultimately leading to chronic kidney disease (CKD).
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Nephrotoxicity
A recent study analyzed the NHANES 2013-2016 data of adolescents who had plasma and water fluoride measurements. Investigators found that each 1 micromole/L increase in plasma fluoride was associated with a 10 mL/min decrease in the estimated glomerular filtration rate (eGFR).
Earlier animal studies have shown that fluoride was nephrotoxic even at low concentrations. Exposed kidneys demonstrated degeneration of tubular cell lining, necrosis, glomerular changes, interstitial swelling, and interstitial nephritis.
Synergy with other toxic elements
Higher level of fluoride in the drinking water was noticed in areas affected by chronic kidney disease of unknown etiology (CKDu). The distribution pattern demonstrated that fluoride could have a synergistic effect on kidney toxicity from cadmium and arsenic.
Oxidative stress
Mitochondrial toxicity and increased oxidative stress were shown in fluorosis. Studies also demonstrated that fluoride kidney toxicity is also mediated by oxidative stress. In fact, animal studies demonstrated a step-wise increase in oxidative stress in the kidneys with increasing levels of fluoride.
Increased risk for kidney stones
Patients who were exposed to higher levels of fluoride were found to be at increased risk for kidney stones. Indeed, fluoride was found to increase calcium and oxalate excretion in the urine. Fluoride was reported to cause renal calcifications due to increased parathyroid hormone levels (PTH).
Fluoride accumulates in kidney disease
On the other hand, studies have shown that fluoride excretion in the urine is decreased in patients with chronic renal disease compared to the normal population. This leads to higher retention of fluoride and increased toxicity. CKD patients were found to develop bone toxicity (skeletal fluorosis) even at one ppm fluoride in the drinking water.
What Can You Do to Protect Your Kidney Health?
If you're concerned about the potential health risks associated with fluoride, there are several things you can do to protect your kidney health:
- Be mindful of your fluoride intake: Be aware of the fluoride content in your drinking water. Check with your local water supplier to find out the fluoride level in your tap water, and consider using a water filtration system if the fluoride level is high. Also, be mindful of the fluoride content in toothpaste and mouthwash, and consider using fluoride-free alternatives if you're concerned about excessive fluoride exposure.
- Stay hydrated: Drinking plenty of water can help flush out toxins and prevent the formation of kidney stones. Aim for at least eight glasses of water a day, and more if you're physically active or live in a hot climate.
- Eat a healthy, plant-dominant diet: Eating a diet rich in fruits, vegetables, whole grains, and protein can help support kidney health. Limit your intake of processed foods, sugary drinks, and foods high in salt and saturated fat.
- Get regular check-ups: If you're concerned about your kidney health, talk to your healthcare provider. They can perform blood and urine tests to assess your kidney function and identify any potential issues early on.
The bottom line
While fluoride is generally considered safe at low levels, there are growing concerns about the potential health risks associated with long-term exposure to high levels of fluoride, particularly in relation to kidney health. By being mindful of your fluoride intake, staying hydrated, eating a healthy diet, and getting regular check-ups, you can help protect your kidney health and reduce your risk of potential health complications.
April Research and News 2023
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney April Research and News.
April Research and News
Higher serum bicarbonate is associated with a decrease in the progression of CKD
Metabolic acidosis describes rising blood acidity (we are not talking about stomach acidity or acid reflux). It occurs in chronic kidney disease (CKD) because of the loss of the kidney's ability to excrete acids.
Several studies demonstrated that metabolic acidosis is associated with bone disease, muscle wasting, and even faster progression of CKD.
This large study published in Kidney Medicine confirms that correcting metabolic acidosis slows the progression of CKD. In this study, investigators studied 51,558 US patients with CKD stage 3-5.
They compared patients with low serum bicarbonate (metabolic acidosis and those with normal serum bicarbonate. They looked at differences between the two groups in outcomes such as a decline in estimated glomerular filtration rate (eGFR), and annual health care cost.
Those with metabolic acidosis had a much higher risk for the progression of CKD after 2 years.
Each 1 mEq/L increase in serum bicarbonate was associated with a 13% decrease in the progression of CKD and a 7% decrease in the annual cost of care.
This is probably the largest study to date that links acidosis to the progression of CKD.
There are lots of other studies that found that correcting metabolic acidosis in CKD is associated with improved outcomes without causing an increase in blood pressure.
By the way, 1 teaspoon has about 60 mEq of bicarbonate compared to only 7.7 in sodium bicarbonate tablets.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Glutathione peroxidase is decreased in CKD and is linked to fibrosis
One of the major problems with the progression of CKD is the development of irreversible fibrosis (scarring).
In this basic research study, researchers looked at the extracellular matrix in mice with CKD. They performed a "proteome-wide" analysis and compared the extracellular matrix in mice with CKD and those who are normal.
They found 9 proteins that could play a role in causing oxidative stress, fibrosis, and inflammation.
However, only glutathione peroxidase 3 was decreased in CKD.
Glutathione peroxidase 3 is responsible for converting glutathione (our master antioxidant) to its oxidized state. See the figure below.
Glutathione helps to recycle other antioxidants. On top of that, GSH has other functions, such as metabolism, cell signaling, and protein interactions, that can also mediate defense against oxidants. It helps the body get rid of heavy metals and a long list of toxins.
A decrease in glutathione peroxidase 3 in CKD that is observed in this study hinders the kidney's ability to deal with oxidative stress, which can lead to inflammation and fibrosis.
Therefore supporting glutathione may be one of the crucial elements in decreasing fibrosis in CKD.
In a population-based study, SGLT2 inhibitors are not as safe as they have been depicted
In a study published in CJASN, investigators looked at healthcare insurance claims data of patients with CKD stage 3-4 and type 2 diabetes who newly filled prescriptions for either sodium-glucose cotransporter-2 inhibitors (SGLT2i) or glucagon-like peptide-1 receptor agonists (GLP-1RA).
They compared safety outcomes between the two groups. These included the development of diabetic ketoacidosis (DKA), lower-limb amputations, non-vertebral fractures, genital infection, hypovolemia (dehydration), acute kidney injury (AKI), hypoglycemia (low blood sugar), and severe urinary tract infections (UTI).
They found that SGLT2i was associated with a higher risk for non-vertebral fractures, lower limb amputations, and genital infections.
The rate of DKA, dehydration and hypoglycemia, and severe UTI were relatively the same in both groups. SGLT2i was associated with a lower risk for AKI.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Benefits of Quercetin to the Kidneys
Quercetin is a flavonoid, a group of plant pigments that have been found to have various health benefits. This natural compound is commonly found in fruits and vegetables such as apples, grapes, onions, berries, and leafy green vegetables. One of the many health benefits of quercetin is its positive effect on kidney function. In this blog, we will discuss the benefits of quercetin to the kidneys.
The benefits of quercetin to the kidneys
By Majd Isreb, MD, FACP, FASN, IFMCP
Anti-inflammatory properties
Quercetin has been found to possess anti-inflammatory properties. Inflammation is a common cause of kidney damage and can lead to chronic kidney disease. Quercetin can reduce inflammation in the kidneys, which may help to prevent or slow down the progression of kidney disease.
Antioxidant properties
Quercetin also has antioxidant properties. Antioxidants are important because they help to neutralize harmful free radicals in the body that can cause damage to cells and tissues. Free radicals can damage the kidneys, leading to kidney disease. By neutralizing these free radicals, quercetin helps to protect the kidneys from damage.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Blood pressure regulation
High blood pressure is a leading cause of kidney disease. Quercetin has been found to have blood pressure-lowering effects. By reducing blood pressure, quercetin can help to prevent kidney damage and slow down the progression of kidney disease.
Reduced proteinuria
Proteinuria is a condition where protein is present in the urine. This can be a sign of kidney damage or disease. Quercetin has been found to reduce proteinuria, which may indicate that it is helpful in protecting the kidneys from damage.
The role of genetics
Genetic variants can affect the metabolism of quercetin by influencing the expression and activity of enzymes involved in its biotransformation. For example, genetic variations in the SULT1A1 and SULT1E1 genes affect the metabolism of quercetin and its metabolite. Studies found that certain genetic variants were associated with altered levels of quercetin and its metabolites in the blood.
COMT (catechol-O-methyltransferase) is an enzyme involved in the metabolism of catecholamines, including dopamine, epinephrine, and norepinephrine. Variants in the COMT gene can affect the activity of the enzyme and, consequently, the metabolism of catecholamines.
One common variant in the COMT gene is the Val158Met polymorphism, which involves a substitution of valine (Val) for methionine (Met) at position 158 of the enzyme. The Val158Met polymorphism has been extensively studied and is associated with altered enzyme activity, with the Val variant associated with higher activity and the Met variant associated with lower activity.
There is evidence suggesting that the Val158Met polymorphism may also affect the metabolism of flavonoids, including quercetin. Individuals with the Val/Val genotype have more active COMT enzyme. Therefore, those with Met/Met genotype should probably be cautious or avoid using quercetin. Those patients will also likely to experience anxiety with quercetin, especially at high doses.
What dose of quercetin should you use?
The recommended dose of quercetin depends on the reason for use, age, sex, and other individual factors. Since quercetin is considered a dietary supplement, there is no standard recommended dose established by the FDA.
However, some studies have used doses ranging from 500 to 1000 mg per day for various health conditions. For example, a study published in the International Journal of Sports Nutrition and Exercise Metabolism used a dose of 1000 mg per day of quercetin supplementation to improve athletic performance in trained cyclists.
A meta-analysis published in the Journal of the American Heart Association found that a dose of 500 mg or more per day of quercetin supplementation improved blood pressure.
It is important to note that high doses of quercetin supplements can cause side effects such as headaches, gastrointestinal discomfort, and kidney damage, especially in those with advanced kidney disease. Quercetin also interacts with a lot of medications.
Therefore, it is recommended to consult a healthcare professional before starting to take quercetin supplements and to follow the dosage instructions on the supplement label.
The use of quercetin in advanced kidney disease
While quercetin has many benefits, as mentioned above, there is limited research on the use of quercetin in advanced kidney disease. The available studies suggest that caution should be taken when considering its use in this population. We recommend avoiding using quercetin for patients with eGFR of less than 30 ml/min.
The bottom line
Quercetin is a natural compound that has numerous health benefits, including its positive effect on kidney function. Quercetin has anti-inflammatory and antioxidant properties, can help to regulate blood pressure, and reduce proteinuria. These benefits make quercetin a promising natural remedy for protecting and maintaining kidney health. However, it is important to consult with a healthcare provider before taking quercetin supplements or making any changes to your diet or medication regimen.
Accuracy of Hemoglobin A1c in CKD
Managing diabetes in patients with chronic kidney disease (CKD) can be challenging, particularly when assessing glucose control. Hemoglobin A1c (HbA1c) testing is the gold standard for monitoring long-term glycemic control in diabetes. However, there are several limitations to the use of HbA1c in CKD. In this blog, I will explore the accuracy of hemoglobin A1c in CKD and discuss alternative tests that may provide more reliable results.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is hemoglobin A1c?
Hemoglobin is a protein found in red blood cells that carries oxygen to the body's tissues. There are different types of hemoglobin, such as A and A2, etc. Hemoglobin A1c (HbA1c) is a form of hemoglobin formed by the non-enzymatic glycation of hemoglobin A with glucose. In essence, when glucose (blood sugar) attaches to hemoglobin, it forms a molecule called glycated hemoglobin, or HbA1c.
HbA1c reflects the average blood glucose levels over the past 2-3 months and is an important tool for managing diabetes. Unlike other blood glucose tests, HbA1c does not require fasting, and the test can be performed at any time of the day.
Moreover, HbA1c provides an integrated measure of blood glucose levels over time, which can help identify long-term glycemic control and the risk of diabetes-related complications.
HbA1c targets
HbA1c testing has become the preferred method for monitoring blood glucose levels in people with diabetes. The American Diabetes Association (ADA) recommends that HbA1c be measured at least twice a year in people with diabetes who are meeting treatment goals and quarterly in people whose therapy has changed or who are not meeting glycemic goals. The target HbA1c level for most adults with diabetes is less than 7%.
Accuracy of hemoglobin A1c in CKD
Challenges of hemoglobin A1c testing in CKD patients
The formation of HbA1c is mainly dependent on the interaction between blood glucose and hemoglobin inside the red blood cells. So, you can see that any factors affecting the red blood cells, hemoglobin, and the glycation process can influence HbA1c. The following factors were documented to affect HbA1c besides blood glucose levels:
- Red blood cell production and lifespan
- The presence of altered hemoglobin
- Interference with the glycation process
- Interference with the assay used
Changes in red blood cells production and lifespan
A Decreased production of red blood cells (RBCs) causes more circulating aged RBCs, for example. This increases hemoglobin contact time with glucose and artificially raises HbA1c. In fact, Iron deficiency anemia was documented to cause an increase in HbA1c of up to 2%. However, this effect was reversed with iron supplementation.
Shortened RBCs lifespan, as it occurs in acute blood loss or enlarged spleen or hemolysis, can decrease hemoglobin exposure time to glucose, causing falsely lowered HbA1c.
In CKD, there are changes in RBCs' lifespan and production. For example, RBC lifespan may be shortened due to the uremic environment, which causes erroneously low HbA1c.
On the other hand, in anemia of chronic kidney disease, there is a decreased production of RBCs which increases the age of circulating RBCs and lengthens exposure to glucose. This will cause artificially elevated HbA1c.
Alteration in hemoglobin
Different assays can give different results in patients who have hemoglobin variants such as hemoglobin S and hemoglobin C. Specialized assays can be used to make these alterations in hemoglobin no longer relevant when measuring HbA1c.
Interference with the glycation process
Interference with the glycation process can lead to artificial changes in HbA1c that are independent of glycemic control. Increased acidity inside the RBCs was found to affect glycation and decrease HbA1c. Increased lipid peroxidation of hemoglobin in CKD has been found to increase its glycation, leading to erroneously higher HbA1c.
Chronic ingestion of aspirin, alcohol, and high doses of antioxidants (like vitamins C and E) can interfere with glycation and falsely lower HbA1c.
Interference with the assay
Finally, it has been reported that high triglyceride, high bilirubin, and opiates can interfere with HbA1c measurements. In CKD, there is carbamylation of hemoglobin due to uremia. Carbamylated hemoglobin also interferes with HbA1c assays causing falsely elevated HbA1c.
Clinical implications
The inaccuracy of HbA1c testing in CKD patients has several clinical implications. Here are some key considerations:
- Misinterpretation of glycemic control: HbA1c levels that are lower than expected in CKD patients may lead to misinterpretation of glycemic control, potentially resulting in under-treatment and increased risk of diabetes-related complications.
- Difficulty in diagnosing diabetes: HbA1c may be less reliable in diagnosing diabetes in CKD patients. Therefore, additional tests such as fasting plasma glucose or oral glucose tolerance test may be needed to confirm the diagnosis.
- Treatment decisions: Treatment based on HbA1c levels alone may not be appropriate in CKD patients.
- Need for an individualized approach: Due to the complex interplay between CKD and HbA1c, a one-size-fits-all approach may not be appropriate for managing diabetes in CKD patients. Instead, an individualized approach, considering other factors such as anemia, iron deficiency, and inflammation, may be needed to assess glycemic control accurately.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Alternative tests for HbA1c in CKD
While HbA1c is the most commonly used marker of glycemic control, its accuracy in CKD patients can be affected by several factors. Therefore, alternative markers of glycemic control may be needed in CKD patients. Here are some markers that may be more reliable in assessing glycemic control in CKD patients:
- Glycated albumin (GA): GA is a marker of short-term glycemic control that reflects the average glucose levels over the preceding 2-3 weeks. GA is not affected by erythrocyte turnover and may be more reliable than HbA1c in CKD patients. Several studies have shown that GA is more accurate than HbA1c in assessing glycemic control in CKD patients. The reference range of GA in Americans with normal glucose tolerance was determined to be 9–15.8%.
- Fructosamine: Fructosamine is another marker of short-term glycemic control that reflects the average glucose levels over the preceding 2-3 weeks. Like GA, fructosamine is not affected by erythrocyte turnover and may be more reliable than HbA1c in CKD patients. The reference range for fructosamine in non-diabetic individuals is 200 to 285 micromol/L. However, there is a lack of standardization across different fructosamine assays.
- Continuous glucose monitoring (CGM): CGM is a technique that provides real-time glucose measurements using a sensor placed under the skin. CGM can provide a more detailed assessment of glycemic control than HbA1c and may be particularly useful in CKD patients with fluctuating glucose levels.
- Self-monitoring of blood glucose (SMBG): SMBG involves regularly measuring blood glucose levels using a glucometer. SMBG may be particularly useful in CKD patients who cannot qualify to get CGM.
The bottom line
Accurate assessment of glycemic control is critical in the management of diabetes in CKD patients. Unfortunately, HbA1c is not a reliable test for glycemic control in this population. An individualized approach, taking into account other factors that may affect HbA1c levels, may be necessary to ensure optimal management of diabetes in CKD patients. Alternative markers of glycemic control should be considered when appropriate.
What Causes FSGS?
FSGS stands for Focal Segmental Glomerulosclerosis, a type of autoimmune kidney disease (glomerulonephritis). It is characterized by scarring of the tiny filtering units in the kidney (glomeruli) and leads to a progressive decline in kidney function. In this blog, I will discuss what causes FSGS.
What Causes FSGS?
By Majd Isreb, MD, FACP, FASN, IFMCP
FSGS is one of the common glomerulonephrites. The symptoms may include swelling, foamy urine, and high blood pressure. Early diagnosis and treatment can help slow the progression of FSGS and prevent kidney failure.
The pathology of FSGS
As in almost all glomerulonephritis, FSGS is described according to the kidney biopsy findings. In FSGS, sclerotic lesions affect parts (focal) of some (segmental) glomeruli.
Based on pathology, FSGS can be divided into several types:
- Tip lesion involves the end or “tip” of the glomeruli. This seems to be the most benign and responsive to therapy.
- The perihilar lesion affects the glomeruli near the renal arterioles.
- Cellular, which presents in the early stages of the evolution of FSGS and is the least common variant.
- Classic (NOS), which is the most common subtype.
- Collapsing FSGS is characterized by severe scarring and damage to the glomeruli, resulting in abrupt proteinuria (excessive protein in the urine) and progressive kidney function decline. This carries the worst prognosis with poor response to conventional treatments.
What causes FSGS?
As you can see, the name FSGS is defined by the histological pattern of glomerular injury. At the root cause, FSGS is not a single disease entity. FSGS, for example, can be caused by various factors, including genetics, infections, drug use, and obesity.
Genetics of FSGS
FSGS can have a genetic component, as some forms of the disease are inherited and run in families. More than 50 genes have been described as potentially responsible for FSGS.
Mutations in genes such as NPHS1 or NHPS2 are associated with the rapid onset of massive protein loss in the urine in early childhood. While mutations or SNPS in other genes have been linked to inherited cases of FSGS that appear later in life. These includes ACTN4, INF2, and TRPC6 genes.
Even genetic variants in the COQ2 gene, which provide instructions for making an enzyme that produces coenzyme Q10 (CoQ10), have been associated with FSGS. Affected individuals were treated successfully with CoQ10 supplementation.
In some cases, the genetic basis of FSGS is still unknown, and further research is needed to fully understand the genetics of the disease. In addition, other environmental, genetic, or epigenetic modifiers could explain the development of FSGS in certain families.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Infections that are associated with FSGS
Various infections have been associated with the development of FSGS. These include:
- HIV/AIDS: Human Immunodeficiency Virus (HIV) can lead to FSGS in some individuals with AIDS.
- Hepatitis B and hepatitis C: Chronic Hepatitis B and C infections can increase the risk of FSGS.
- Other viruses, such as parvovirus B19 and Cytomegalovirus, can cause FSGS.
- Streptococcal infections: Repeat streptococcal infections, such as strep throat, have been linked to FSGS in some cases.
- Lyme disease: The bacterium that causes Lyme disease, Borrelia burgdorferi, can trigger a disease called minimal change disease (MCD) in some individuals. MCD is another glomerulonephritis, and it could be argued that it is part of the FSGS spectrum of diseases. However, I could not find any case report or review in the literature showing a clear association between Lyme disease and FSGS. If you know of any, could you send them my way?
It's important to note that while infections can trigger FSGS in some cases, not all individuals with these infections will develop FSGS.
Medications and FSGS
Certain medicine has been linked to the development of FSGS, these includes:
- Nonsteroidal anti-inflammatory drugs (NSAIDs): Long-term use of NSAIDs, such as ibuprofen, have sometimes been linked to FSGS.
- Interferon
- Bisphosphonates
- Anabolic steroids
- Lithium
- Sirolimus
- Tyrosine kinase inhibitors
- Immune checkpoint inhibitors (Nivolumab, Pembrolizumab, and Ipilimumab)
- Street drugs: Illegal street drugs, such as cocaine and heroin, have been associated with FSGS.
- Supplement?
- Herbs?
It's important to note that while drug use can trigger FSGS in some cases, not all individuals who take these drugs will develop FSGS. The exact mechanisms behind the association between drug use and FSGS are still being studied. Additionally, the benefits of taking these drugs to treat medical conditions may outweigh the potential risks in some cases.
Maladaptive FSGS
Maladaptive FSGS is usually the result of a “mismatch” between the load on the filtering units of the kidneys and their capacity. This can be seen in situations where the kidney mass is decreased or where there is an increase in waste production. Therefore, any kidney disease that leads to a decrease in the number of filtering units (nephrons) can ultimately cause some form of maladaptive FSGS.
Decreased kidney mass (reduced nephron numbers)
This can be seen in these conditions:
- Reflux nephropathy, in which the urine backs up into the kidneys, causing damage and fewer nephrons.
- Surgical resection of part of the kidneys or one of the kidneys.
- Renal dysplasia is usually congenital and leads to a small kidney.
- The congenital absence of one of the kidneys.
- A decrease in the number of nephrons at birth (nephron endowment)
Normal kidneys but excessive waste production
As in individuals with:
- Obesity
- High intake of protein
The bottom line
FSGS is not a single disease entity. It is defined by the histological pattern of glomerular injury. Various causes can lead to FSGS, including genetics, infections, drug use, and obesity. It is, therefore, essential to look for the root cause of this disease through an Integrative approach. This approach can be made in conjunction with conventional medical therapy, especially in the aggressive forms of this disease.
March Research and News 2023
We combed through multiple medical journals looking for the latest research on the Integrative approach to kidney health. We know your time is valuable, so we curated and summarized these studies for you. Welcome to the InKidney March Research and News.
March Research and News
Another Coffee study, but this one makes sense
Every few months, studies come out investigating the link between coffee consumption and various health outcome. Most studies pool all individuals together and look into the amount of coffee drinking and a specific outcome.
Not this one!
Caffeine is metabolized by cytochrome P450 1A2 (CYP1A2), and genetic variation in CYP1A2 impacts the rate of caffeine clearance.
Some people are fast metabolizers, while others are slow metabolizers. This is why pooling individuals together in research studies may lead to different and sometimes contradictory results.
During 7.5 years of follow-up of 604 untreated adults with stage 1 hypertension from the Hypertension and Ambulatory Recording Venetia Study, those with genetic variants in CYP1A2 who were slow metabolizers of caffeine and had heavy coffee intake (>3 cups/day) were:
- 2.7 times more likely to develop albuminuria,
- 2.5 times more likely to develop hyperfiltration
- 2.8 times more likely to develop hypertension
No associations were observed between coffee intake and albuminuria, hyperfiltration, or hypertension among fast metabolizers of caffeine.
So, the answer to "does drinking coffee affects my kidneys?" is: it depends on your genes.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The mind-body connection in CKD
In a study published in Kidney360, investigators analyzed data from 2,361 individuals with mild to moderate chronic kidney disease (CKD) in the CRIC study. They looked at the link between depression and CKD progression.
They used the Beck Depression Inventory (BDI) to stratify the patients into three groups: persistently low BDI score, persistently moderate BDI score, and persistently high BDI score.
A higher BDI score means worse depression.
They found that moderate and high BDI scores were associated with a rapid decline in kidney function (eGFR).
Low educational attainment, cigarette smoking, and poor quality of life were associated with higher BDI scores.
You can download your own BDI from this link.
Hypertension and inflammation: exploring the link
This review article was published in Current Opinion in Nephrology and Hypertension. It explored the role of inflammation in hypertension and end-organ damage.
The review highlights how immunomodulatory interventions improve outcomes in hypertensive patients independent of arterial pressure. This is especially true for studies targeting interleukin IL-1β, IL-6 and IL-17.
Studies that looked at other markers (αvβ-3 integrin and matrix metalloproteinase-2) found that modulating them can enhance nitric oxide production and decrease endothelial inflammation.
How can you use this in practice?
1. Assessing for inflammation in your patients with hypertension. This can be done by using a simple marker such as the neutrophil-to-lymphocyte ratio (NLR). More advanced markers, such as IL-6, can also be measured.
2. Help patients decrease inflammation by eating more anti-inflammatory food, exercising, getting proper dental care, and managing stress.
3. Increase nitric oxide with a diet rich in vegetables and antioxidants and even supplements such as L-arginine or L-citrulline.
Join here to receive FREE monthly updates on the latest research in Integrative Nephrology and tips on managing kidney disease straight to your inbox.
We would love to hear your feedback. Let us know what you think of these educational materials and if you like us to focus on specific topics. Please email us at info@inkidney.com.
The Role of Integrative Health Coaches
Integrative health coaches play a crucial role in the approach to chronic kidney disease, helping patients navigate their healthcare journeys and empowering them to make informed decisions about their health. As healthcare becomes more complex, the need for integrative health coaches has grown, and research has shown that their work can have a positive impact on patient outcomes.
By Majd Isreb, MD, FACP, FASN, IFMCP
What is an Integrative Health Coach?
An integrative health coach is a trained professional who works with patients to identify their health goals. They work with other Integrative health professionals to develop a personalized plan for each patient.
Integrative health coaches are typically trained in the basics of a range of holistic health practices, including nutrition, stress management, and mindfulness, and work to help patients address the root causes of their health concerns.
It is crucial to know, though, that coaches cannot prescribe medications. Their work does not replace the role of an Integrative-trained dietitian or nutritionist. Ideally, they are part of a team that includes a provider, a dietitian or nutritionist, and a coach.
Role of Integrative Health Coaches
Integrative health coaches are part of a team that develops holistic approaches to managing chronic conditions. Here are a few specific ways that integrative health coaches can support medicine management:
- Provide the patient with tools and skills to help implement lifestyle changes prescribed by their provider and build new habits to improve their overall health.
- Guide them through setting goals and taking steps to achieve them.
- Hold the patient accountable to achieve their goals.
- A health coach will assist the patient in exploring gaps between their current condition and desired goals.
- They may also improve medication adherence if needed and manage side effects.
The Impact of Integrative Health Coaching
Research has shown that integrative health coaching can have a positive impact on patient outcomes. For example, a study published in the Annals of Family Medicine found that integrative health coaching was associated with improvements in glucose control, blood pressure, cholesterol levels, and self-reported health status in patients with metabolic syndrome.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
Another study published in the Journal of the Academy of Nutrition and Dietetics found that integrative health coaching was associated with a decrease in healthcare costs, with participants who received coaching experiencing lower medical expenses than those who did not.
These studies and others suggest that integrative health coaching can be a valuable tool in medicine management, helping patients to take a more active role in their healthcare and achieve better health outcomes.
The bottom line
Integrative health coaches play an important role in the Integrative approach to kidney health, helping patients navigate their healthcare journeys and empowering them to take control of their health. Their work can have an additive positive impact on patient outcomes, and research has shown that integrative health coaching is associated with improvements in a range of health measures.
The role of sex hormones in kidney health
Testosterone and estrogen make up the main sex hormones in men and women, respectively. They play a key role in many bodily functions but are most notably responsible for their role in reproduction and menopause (andropause for men). Today, I will explain the role of sex hormones in kidney health and their protective and destructive role in CKD progression.
The Good, the Bad, and the Sex Hormones; Implications on Kidney Health and CKD Progression
It is well known that women have a higher rate of CKD, but men have a faster rate of CKD progression than women. So how do testosterone, Sex Hormone Binding Globulin (SHBG), and estrogen fit into that? Let’s discuss…
Men vs. Women in Aging and How it Affects Kidneys/Hormones
As we age, the levels of testosterone and estrogen naturally decline, leading to what many know as menopause and andropause. However, many confounding factors play a role in their decline, for example, diet, lifestyle, and comorbidities. Because of this, it has been difficult for researchers to narrow down the exact causal relationship between those factors, the decline in hormone levels, and kidney health.
Despite unanswered questions, researchers have found that testosterone is profibrotic and proinflammatory, which could be one of the reasons why CKD progresses faster in men compared to women.
Indeed, testosterone has been linked to the development and progression of kidney disease in some studies. In addition, high testosterone levels have been associated with decreased GFR and increased proteinuria (excess protein in the urine), both markers of kidney dysfunction.
On the other hand, estrogen has been found to decrease the buildup of the glomerular extracellular matrix and slow the development of glomerulosclerosis. In addition, Estrogen has been shown to have a protective effect on the kidneys, improving glomerular filtration rate (GFR) and reducing proteinuria, markers of kidney function. Estrogen has also been associated with a reduction in the progression of kidney disease in some studies.
Effects on kidney circulation
The renin-angiotensin-aldosterone system is primarily associated with blood pressure regulation by modulating blood volume, sodium reabsorption, potassium secretion, water reabsorption, and vascular tone. Estrogen and testosterone play contrasting roles in the renin-angiotensin-aldosterone system (RAAS).
Estrogen stimulates bradykinin synthesis, which leads to an increase in nitric oxide release. Nitric oxide helps slow the progression of renal disease. Testosterone has the opposite effect on the RAAS; it stimulates it, leading to decreased nitric oxide levels and vasodilatory responses.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
How does SHBG fit into the picture?
Sex hormone-binding globulin (SHBG) is a protein produced in the liver that binds and carries testosterone and estradiol in blood circulation. Doing so regulates the availability of the free forms of the two sex hormones.
Estradiol stimulates the production of SHBG by the liver. SHBG carries protein for testosterone and dihydrotestosterone (DHT). DHT is another androgen hormone that is made from testosterone. The higher the SHBG levels, the less testosterone and DHT are available for the body to use.
As men age, the levels of Estrogen increase, leading to increased production of SHBG and, thus, decreased levels of testosterone. This explains why higher SHBG levels were linked to a lower risk of CKD in men but not women.
In addition, observational studies have shown that low SHBG concentration is associated with an increased incidence of type 2 diabetes mellitus (DM) independent of sex hormone levels in men and women. Furthermore, lower levels of SHBG have been linked to increased insulin resistance. This can be another link between SHBG and the development of kidney disease.
The role of diet
How can you promote hormone health to help improve your kidney health? It starts with the basics, lifestyle, and diet. The benefits of limiting protein in the diet to promote kidney health have been discussed, but how does that fit in with hormones?
A low-protein diet also promotes increased estrogen and decreased testosterone levels in both men and women (Hertoghe, 2010). However, this has been contested in other research studies. Yet, a diet high in fiber and unsaturated fat can be associated with decreased testosterone levels. In addition, recent studies have shown that a plant-dominant low-protein diet has better health outcomes for patients with CKD and other comorbidities like diabetes versus the previously recommended low-protein diet.
This doesn’t mean you should convert to veganism overnight but talk to your doctor about what would be best for your overall health and start by making small changes, i.e., two plant-based meals a week and work up from there.
The bottom line
In the end, there is still a lot for researchers to learn about the causal relationship between sex hormones and CKD progression. Higher testosterone is associated with faster progression of CKD, while estrogen is protective. However, it's important to note that the relationship between sex hormones and kidney diseases is complex and not fully understood. To explore this, talk to your Integrative or Functional Medicine provider to optimize your hormone balance through nutrition and lifestyle modifications.
Glomerulonephrites: The autoimmune kidney diseases
Autoimmune kidney diseases are a group of conditions where the body's immune system mistakenly attacks and damages the kidneys. This leads to inflammation and, eventually, kidney failure. Glomerulonephrites (pleural) are autoimmune kidney diseases.
By Majd Isreb, MD, FACP, FASN, IFMCP
Glomerulonephrites (GN) are the world's third leading cause of kidney disease and failure. It is a group of autoimmune kidney diseases named according to the site they involve in the kidneys and how they appear under the microscope.
Types of glomerulonephrites
GNs are generally classified into “primary” or “secondary.” In primary GN, the kidney is the only apparent site of autoimmune injury. While in secondary GN, kidney injury is one of the manifestations of a systemic autoimmune or non-autoimmune disease. Most of these GNs can be acute or chronic.
The most common primary glomerulonephrites include:
- IgA nephropathy: Also known as Berger's disease, this is the most common type of autoimmune kidney disease. It occurs when immunoglobulin A (IgA) deposits in the kidneys and triggers an immune response.
- Focal Segmental glomerulosclerosis (FSGS): This is characterized by scarring of the tiny filtering units in the kidney (glomeruli) and leads to a progressive decline in kidney function.
- Membranous nephropathy occurs when the immune system attacks the thin layer of tissue (basement membrane) that separates the glomerular filtration barrier.
- Mesangioproliferative GN (MPGN): This is an uncommon kidney disorder characterized by the proliferation (growth) of specific glomerular cells and structural changes in glomerular capillary walls and basement membrane.
- Minimal change disease: In this disorder, the kidney biopsy shows normal findings on a light microscope, but detailed examination shows minor changes in specific glomerular filtering cells under an electron microscope. Some consider this disease on the same spectrum as FSGS.
- Rapidly progressive GN (RPGN): This is an aggressive form of GN seen in vasculitis and can lead to rapid loss of kidney function within days to weeks.
Join us to end the kidney disease epidemic and receive the FREE Report “5 Pitfalls to Avoid When Caring for Kidney Patients”
The most common secondary glomerulonephrites include:
- Postinfectious glomerulonephritis
- Systemic vasculitis
- Lupus nephritis
- Monoclonal gammopathy of renal significance
- Thrombotic microangiopathy
- HIV-related nephropathy
Many primary glomerulonephrites can be a pathological presentation of a systemic process.
Symptoms of Autoimmune Kidney Diseases
Injury to the glomeruli leads to various symptoms and signs. For example, protein can appear in the urine (proteinuria) due to protein leak through the capillary walls of the glomeruli. Blood can also appear in the urine (hematuria) due to ruptured capillaries.
The injury can also decrease the clearance of waste products by the kidneys leading to the accumulation of various toxins. It can lead to a decrease in urine output. Salt and water retention can occur, leading to swelling (edema). Blood pressure can also rise due to disturbed renal balance of blood pressure.
Therefore, it is essential to think of glomerulonephrites when there:
- Dark urine (tea-colored urine) or blood in the urine.
- Excessively foamy or bubbly urine (a sign of excessive protein in the urine)
- Swelling in the legs (especially in the evening) and or the face (especially in the morning)
- Frequent nighttime urination
- Elevated blood pressure
- Symptoms of decreased clearance of waste products such as nausea and vomiting, poor appetite, fatigue, itching, insomnia, and muscle cramps.
Several serological tests can point to one type of GN or another. However, the diagnosis is usually confirmed by a kidney biopsy.
What causes autoimmune kidney diseases?
The triggers for autoimmune kidney diseases are not fully understood, but some factors that are believed to play a role include:
- Genetics: A family history of autoimmune diseases increases the likelihood of developing an autoimmune kidney disease. Certain genetic variants have been associated with certain types of GN.
- Environmental triggers: Certain infections, toxins, or medications can trigger an autoimmune response and contribute to the development of autoimmune kidney diseases.
- Hormonal imbalances: Hormonal changes can lead to a flare-up of autoimmune kidney diseases in some individuals.
- Certain foods: some food such as gluten, soy, or dairy has been associated with GN in certain people.
- Dysbiosis: An increasing body of evidence links dysbiosis with various autoimmune kidney diseases.
In addition, some mediators may worsen autoimmune kidney diseases, such as diet, nutritional deficiencies, psychological stress, and lack of sleep.
The bottom line
Diagnosis of autoimmune kidney diseases is typically based on a combination of medical history, physical exam, and laboratory tests. This may include a urine test to check for protein, blood tests to assess kidney function, and a biopsy to confirm the diagnosis.
Treatment for autoimmune kidney diseases depends on the type and severity of the condition. The integrative approach depends on identifying the predisposing factors and triggers to GN. It manages various mediators and the inflammation associated with them. This approach parallels the conventional method often needed to extinguish some of these aggressive GN.